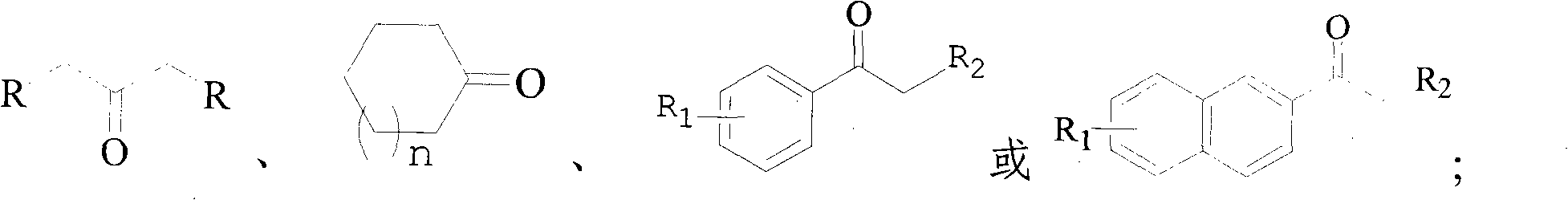
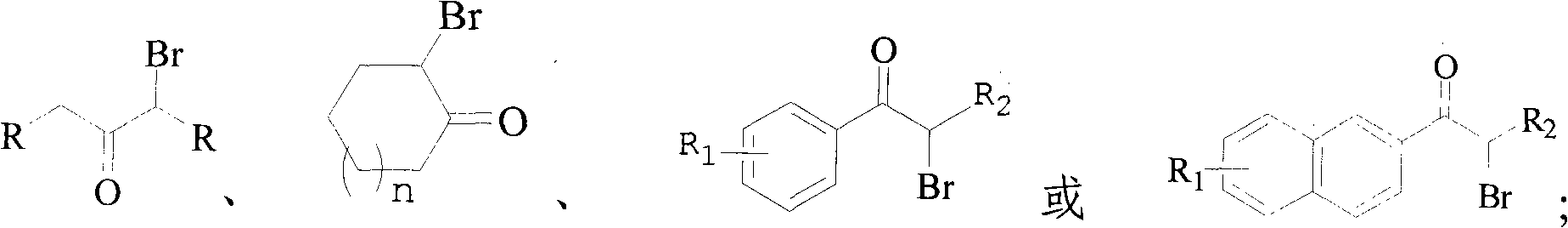
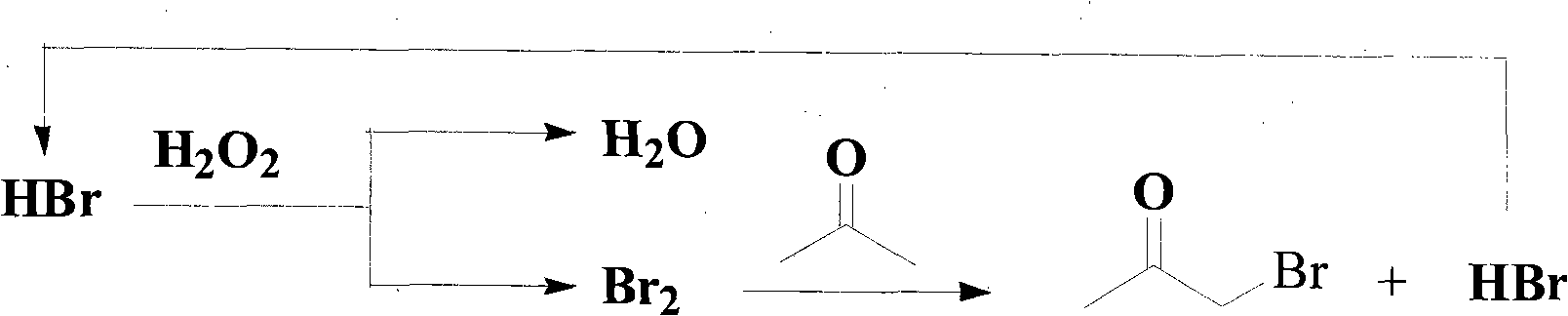Method of synthesizing alpha-brominated ketone compound by hydrogen peroxide oxidizing and brominating method
An oxidative bromination method and a technology for ketone compounds, which are applied in the preparation of organic compounds, the preparation of carbon-based compounds, chemical instruments and methods, etc., can solve problems such as no further utilization of hydrogen bromide, and achieve low cost and convenient operation. , to avoid the effect of corrosion on the equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1. hydrogen bromide-hydrogen peroxide oxidative bromination method synthesizes brominated acetone
[0048]
[0049] Install a stirrer, a thermometer, a long reflux condenser and a feeding tube with a Y-shaped tube at the top of the four-necked flask. The two branches of the Y-shaped tube are each connected to a dropping funnel, and 29g (0.5 mol) acetone and 40mL water, respectively put into two dropping funnels 84.4g (0.5mol) 48% hydrobromic acid and 57g (0.5mol) 30% hydrogen peroxide solution, then add a few drops of hydrogen into the flask under stirring Bromic acid, then add a few drops of hydrogen peroxide dropwise, orange-red bromine will appear in the flask soon, slowly heat to 60-65°C, after the bromine completely fades, continue to drop hydrobromic acid, and then add hydrogen peroxide dropwise. Each time the reagent is repeatedly added dropwise, the bromine produced in the previous batch should basically fade, even if the color of the reactant rema...
Embodiment 2
[0050] Embodiment 2. Hydrogen bromide-hydrogen peroxide oxidative bromination method synthesizes 2-bromo-3-pentanone
[0051]
[0052] Install a stirrer, a thermometer, a long reflux condenser and a feeding tube with a Y-shaped tube at the top of the four-necked flask. The two branches of the Y-shaped tube are each connected to a dropping funnel. Add 43g (0.5 mol) 3-pentanone, 40mL water, 84.4g (0.5mol) 48% hydrobromic acid and 57g (0.5mol) 30% hydrogen peroxide solution are respectively charged in two dropping funnels, then add in the flask under stirring Add a few drops of hydrobromic acid, then drop a few drops of hydrogen peroxide, orange-red bromine will appear in the flask soon, heat slowly to 55-60°C, continue to drop hydrobromic acid after the bromine has completely faded, and then add dropwise hydrogen oxide. Each time the reagent is repeatedly added dropwise, the bromine produced in the previous batch should basically fade, even if the color of the reactant remai...
Embodiment 3
[0053] Embodiment 3. Hydrogen bromide-hydrogen peroxide oxidative bromination method synthesizes α-bromocyclohexanone
[0054]
[0055] Install a stirrer, a thermometer, a long reflux condenser and a feeding tube with a Y-shaped tube at the top of the four-necked flask. The two branches of the Y-shaped tube are each connected to a dropping funnel. Add 49g (0.5 mol) cyclohexanone, 40mL water, respectively load 84.4g (0.5mol) 48% hydrobromic acid and 57g (0.5mol) 30% hydrogen peroxide solution in two dropping funnels, then add several Drop hydrobromic acid, then add a few drops of hydrogen peroxide, orange-red bromine will appear soon in the flask, slowly heat to 50-60°C, continue to drop hydrobromic acid after the bromine has completely faded, and then add dropwise peroxide hydrogen. Each time the reagent is repeatedly added dropwise, the bromine produced in the previous batch should basically fade, even if the color of the reactant remains in an orange-yellow state. Repea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com