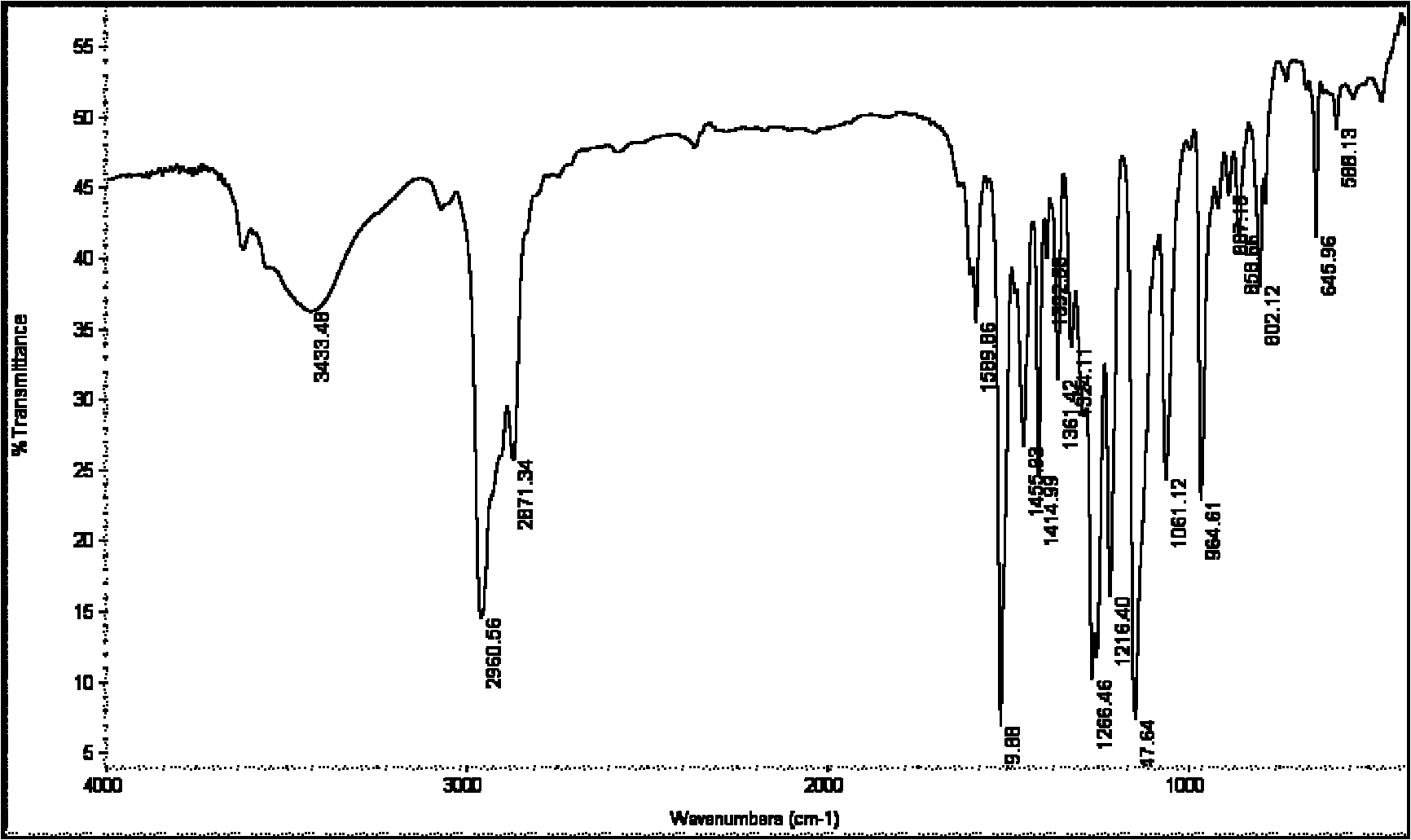
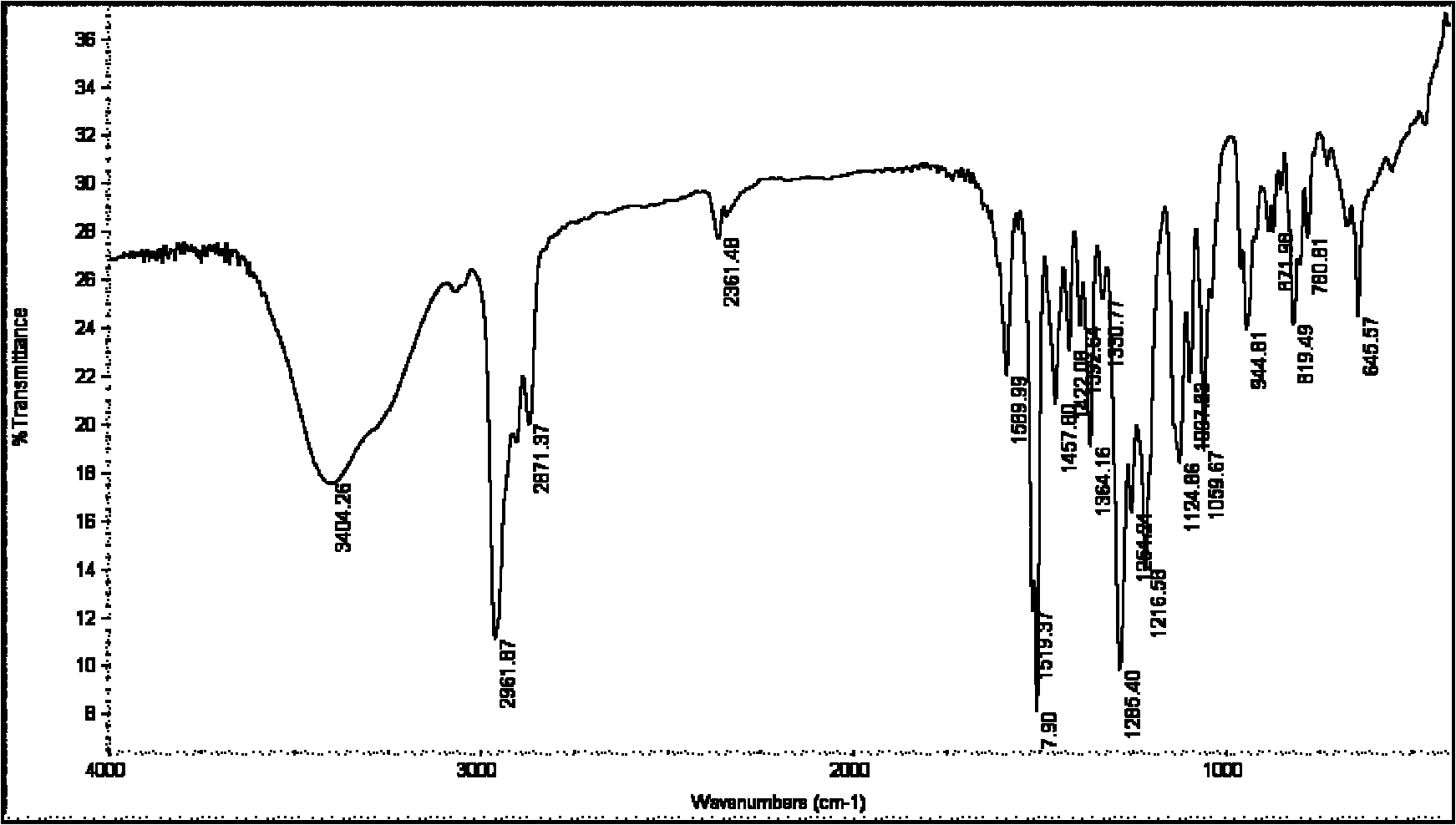
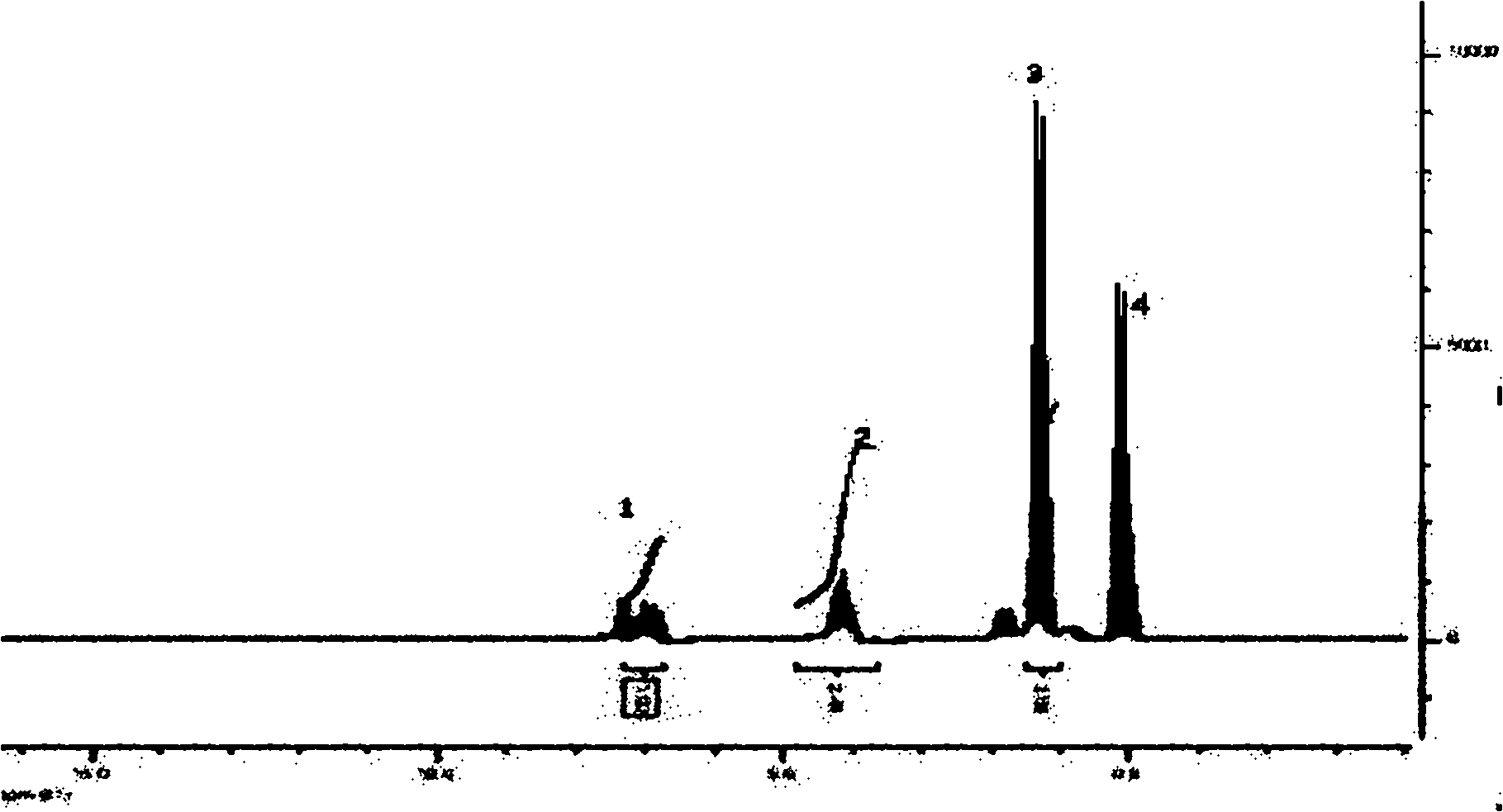
Preparation technology of 4'4'' (5'')-di-tert-butyl-di-benzo-18-crown-6
A technology for the preparation of di-tert-butyldibenzo, which is applied in the field of organic synthesis, can solve the problems of difficult removal of impurities, long time, cumbersome decomplexation process, etc., to reduce solvation, increase conversion rate, and increase concentration and the effect of cationic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a. Weigh 8.3g (0.05mol) of p-tert-butylcatechol and 35.8g (0.11mol) of cesium carbonate and dissolve them in 500ml of tetrahydrofuran, wherein the cesium carbonate is added in two equal amounts. A certain amount of diethylene glycol xylene sulfonate was weighed, dissolved in 250ml tetrahydrofuran twice, and added dropwise in a constant pressure dropping funnel. The reaction was carried out at 60°C for 48 hours, and the reaction conversion rate was 52.3%.
[0032] b. Filtrate the mixture obtained by reacting according to the above step a, and take the filtrate for vacuum distillation. The residue was dissolved in methanol, filtered, and the filtrate was vacuum distilled. Dichloromethane elutes the residue, washes repeatedly with 1N HCl and hot sodium thiosulfate aqueous solution, separates the organic phase, and dries it with anhydrous magnesium sulfate for use.
[0033] c. Weigh 5 g of the mixture treated according to the above step b, add 250 g of basic alumina and 5...
Embodiment 2
[0035] a. Weigh 8.3g (0.05mol) of p-tert-butylcatechol and 37.6g (0.115mol) of cesium carbonate and dissolve them in 400ml of tetrahydrofuran, wherein the cesium carbonate is added in two equal amounts. A certain amount of diethylene glycol xylene sulfonate was weighed, dissolved in 200ml tetrahydrofuran twice, and added dropwise in a constant pressure dropping funnel. The reaction was carried out at 50°C for 36 hours, and the reaction conversion rate was 43.8%.
[0036] b. Filtrate the mixture obtained in step a above, and get the filtrate for vacuum distillation. Methanol strips the residue, continues to filter, and then takes the filtrate for vacuum distillation. Dichloromethane elutes the residue, washes repeatedly with 1N HCl and hot sodium thiosulfate aqueous solution, separates the organic phase, and dries it with anhydrous magnesium sulfate for use.
[0037] c. Weigh 100 g of 200-300 mesh column chromatography silica gel, and dry-pack the column. Weigh 1 g of the mi...
Embodiment 3
[0039] a. Weigh 8.3g (0.05mol) of p-tert-butylcatechol and 40.6g (0.125mol) of cesium carbonate and dissolve them in 300ml of tetrahydrofuran, wherein the cesium carbonate is added in two equal amounts. A certain amount of diethylene glycol xylene sulfonate was weighed, dissolved in 150ml tetrahydrofuran twice, and added dropwise in a constant pressure dropping funnel. The reaction was carried out at 40°C for 24 hours, and the reaction conversion rate was 33.7%.
[0040] b. Filtrate the mixture obtained in step a above, and get the filtrate for vacuum distillation. Methanol strips the residue, continues to filter, and then takes the filtrate for vacuum distillation. Dichloromethane elutes the residue, washes repeatedly with 1N HCl and hot sodium thiosulfate aqueous solution, separates the organic phase, and dries it with anhydrous magnesium sulfate for use.
[0041] c. Weigh 60 g of 200-300 mesh column chromatography silica gel, and dry-pack the column. Weigh 1 g of the mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com