Method for preparing flaky calcite calcium carbonate crystal
A calcite, calcium carbonate technology, applied in the direction of calcium carbonate/strontium/barium, crystal growth, chemical instruments and methods, etc., can solve the problems of complex process, large particle size of calcium carbonate, uneven distribution, etc., to achieve simple process, The effect of uniform particle size and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
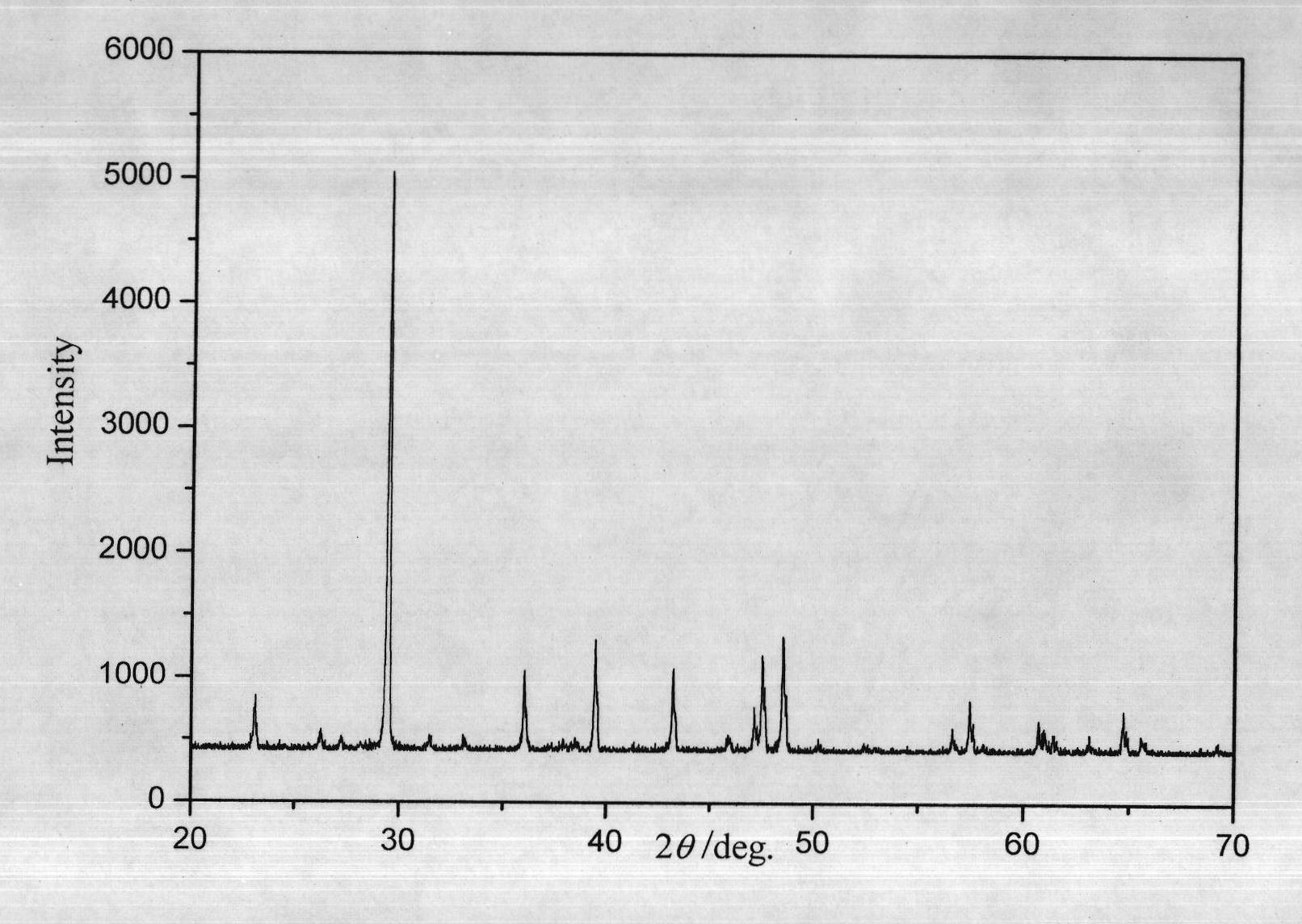
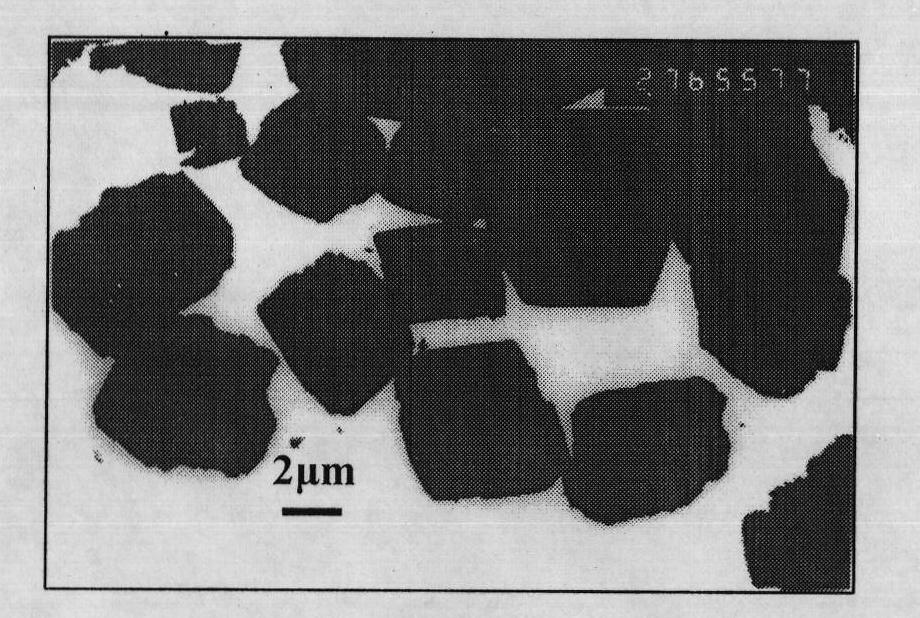
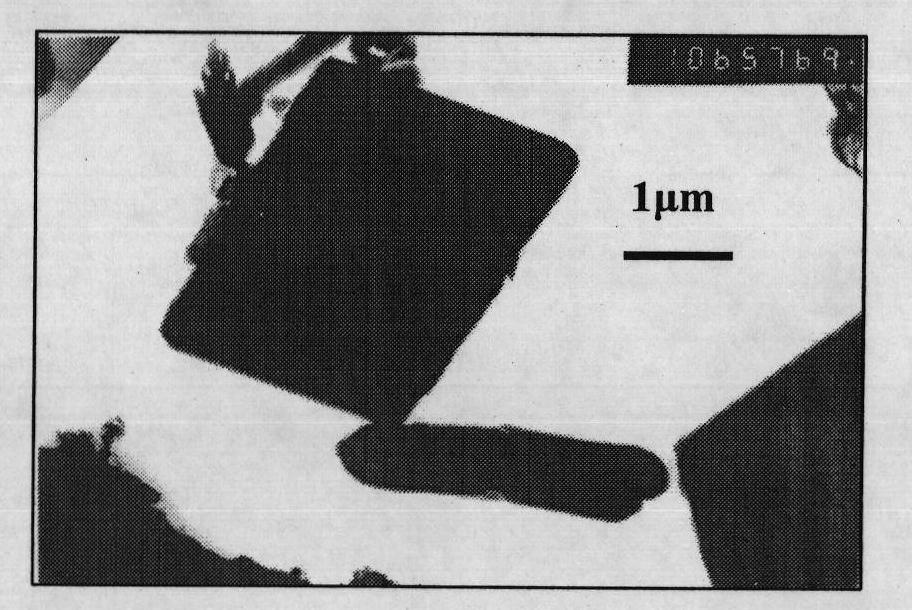
Image
Examples
Embodiment 1
[0021] Embodiment 1: the mass ratio of calcium chloride and sodium carbonate is 1.4: 1
[0022] (1) in the sodium carbonate solution that 63mL concentration is 0.52mol / L, add the boric acid that is sodium carbonate quality 0.8% as crystal formation control agent, control solution temperature is 40 ℃;
[0023] (2) Take 37 mL of calcium chloride solution with a concentration of 1.24 mol / L, and control the temperature of the solution to be 40° C.;
[0024] (3) Add the calcium chloride solution dropwise to the sodium carbonate solution at a rate of 0.5mL / min under the conditions of a stirring speed of 200r / min and a temperature of 40°C. Continue to stir at constant temperature for 0.5h, then stand at room temperature for 12h, and filter to obtain a precipitate;
[0025] (4) Repeatedly wash the precipitate with deionized water to neutrality, remove the chloride ions adsorbed on the surface of the precipitate through washing, filter, and vacuum-dry at 40° C. for 4 hours to obtain t...
Embodiment 2
[0027] Embodiment 2: the mass ratio of calcium chloride and sodium carbonate is 1.6: 1
[0028] (1) adding oxalic acid that is 2.2% of the quality of sodium carbonate as a crystal form control agent in 54mL of sodium carbonate solution with a concentration of 1.6mol / L, and controlling the solution temperature to be 55°C;
[0029] (2) Take 46 mL of calcium chloride solution with a concentration of 2.9 mol / L, and control the temperature of the solution to be 55° C.;
[0030] (3) Add the calcium chloride solution dropwise to the sodium carbonate solution at a rate of 0.8mL / min under the condition that the stirring speed is 300r / min and the temperature is 55°C. Continue to stir at constant temperature for 1h, then stand at room temperature for 20h, and filter to obtain a precipitate;
[0031] (4) Repeatedly wash the precipitate with deionized water to neutrality, remove the chloride ions adsorbed on the surface of the precipitate through washing, filter, and vacuum dry at 55° C. ...
Embodiment 3
[0033] Embodiment 3: the mass ratio of calcium chloride and sodium carbonate is 1.7: 1
[0034] (1) in the sodium carbonate solution that 50mL concentration is 2.7mol / L, add the boric acid that is sodium carbonate quality 3.5% and make crystal formation control agent, control solution temperature is 70 ℃;
[0035] (2) Take 50 mL of calcium chloride solution with a concentration of 4.6 mol / L, and control the temperature of the solution to be 70° C.;
[0036] (3) Add the calcium chloride solution dropwise to the sodium carbonate solution at a rate of 1.2mL / min under the condition that the stirring speed is 400r / min and the temperature is 70°C. Continue to stir at constant temperature for 1.5h, then stand at room temperature for 24h, and filter to obtain a precipitate;
[0037] (4) Repeatedly wash the precipitate with deionized water to neutrality, remove the chloride ions adsorbed on the surface of the precipitate through washing, filter, and vacuum dry at 70° C. for 2.5 hours ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com