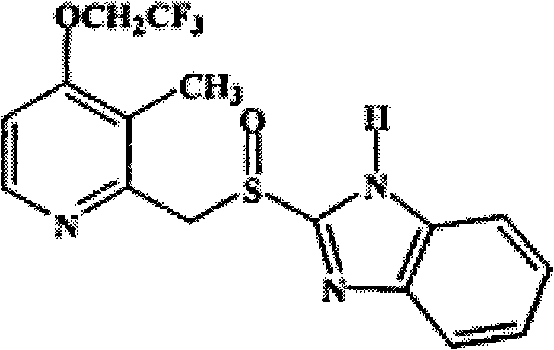
Lansoprazole enteric preparation and preparation method thereof
A technology of lansoprazole enteric and enteric coating, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of low dissolution rate and low dissolution rate of lansoprazole , low dissolution rate and other issues, to achieve the effects of low economic cost, beneficial to human safety, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
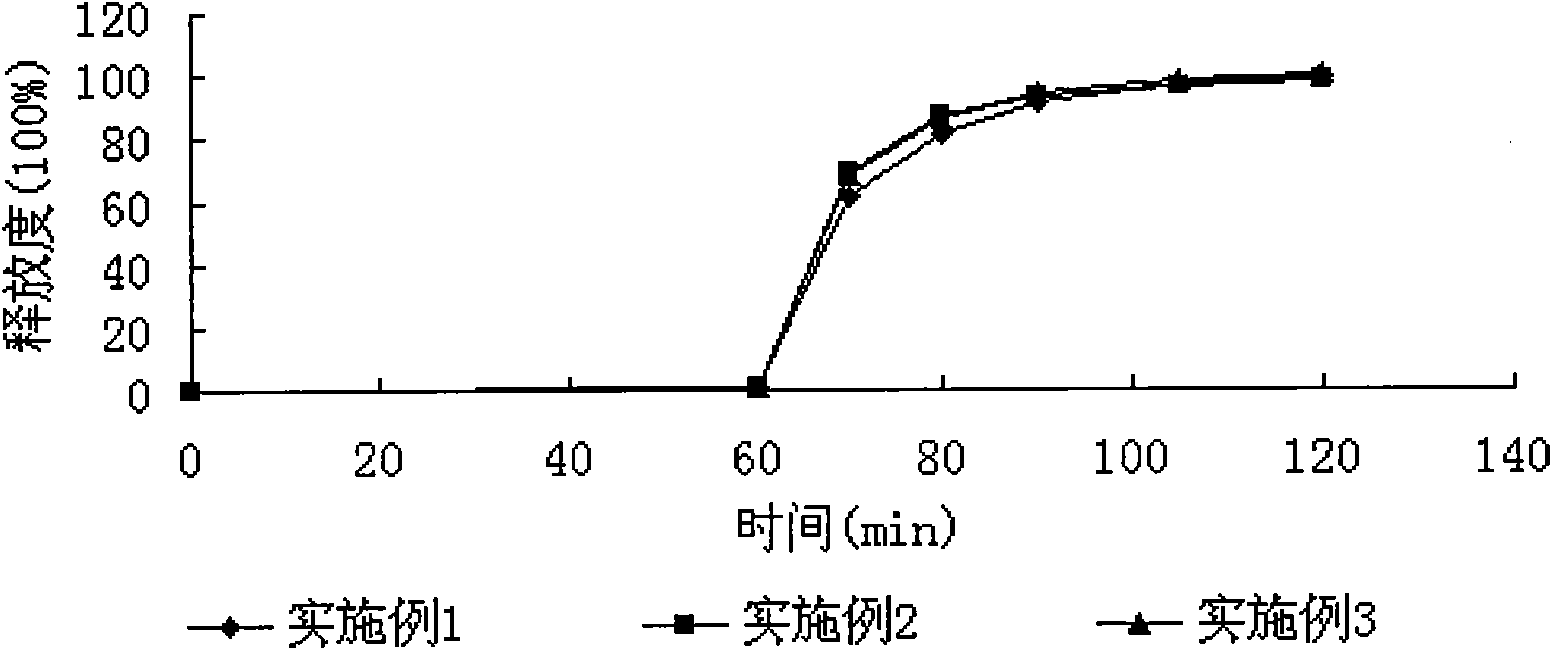
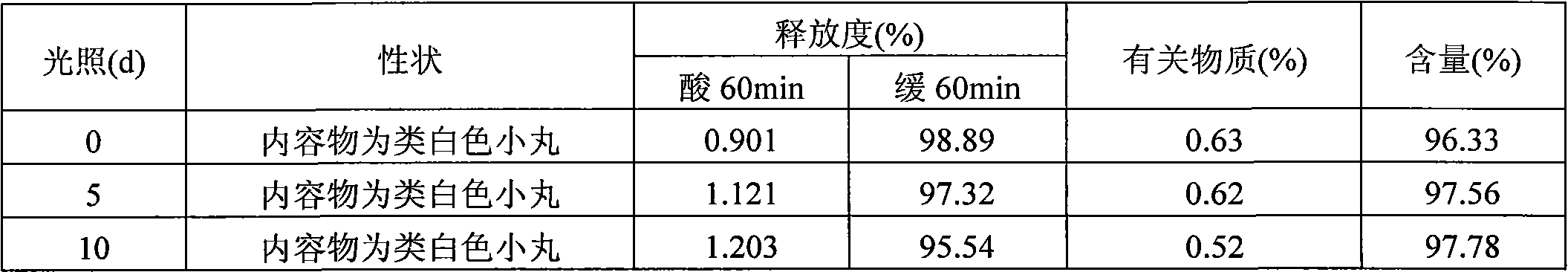
Examples
Embodiment 1
[0041] Drugs and preparatory excipients
[0042] (1) drug layer
[0043] Blank ball core 80.0g
[0044] Lansoprazole 20.0g
[0046] Poloxamer 188 4.0g
[0047] Crospovidone 2.5g
[0048] Polyvinylpyrrolidone 10.0g
[0049] Proper amount of ethanol
[0050] (2) isolation layer
[0052] Titanium dioxide 9.0g
[0053] Polyvinylpyrrolidone 10.0g
[0054] Anhydrous ethanol amount
[0055] (3) Enteric coating layer
[0056] Udrake L100-55 60.0g
[0057] PEG40006.0g
[0059] Anhydrous ethanol amount
[0060] Preparation:
[0061] (1) Drug layer preparation
[0062] Get an appropriate amount of ethanol, slowly add magnesia, polyvinylpyrrolidone, crospovidone and poloxamer that are sieved and mixed, stir evenly, add the lansoprazole crude drug to obtain a drug suspension, and spray the drug suspension to Blank ball core surface, get medicine ball core;
[0063] (2) Isolation gown prepa...
Embodiment 2
[0086] Drugs and preparatory excipients
[0087] (1) drug layer
[0088] Blank ball core 80.0g
[0089] Lansoprazole 20.0g
[0090] Magnesium carbonate 2.5g
[0091] Poloxamer 188 4.0g
[0092] Crospovidone 2.5g
[0093] Polyvinylpyrrolidone 10.0g
[0094] Proper amount of ethanol
[0095] (2) isolation layer
[0097] Titanium dioxide 9.0g
[0098] Polyvinylpyrrolidone 10.0g
[0099] Anhydrous ethanol amount
[0100] (3) Enteric coating layer
[0101] Udrake L100-55 60.0g
[0102] PEG40006.0g
[0103] Talc powder 15.0g
[0104] Anhydrous ethanol amount
[0105] Preparation:
[0106] (1) Drug layer preparation
[0107] Get an appropriate amount of ethanol, slowly add sieved and mixed magnesium carbonate, polyvinylpyrrolidone, crospovidone and poloxamer, stir evenly, add the lansoprazole crude drug to obtain a drug suspension, and spray the drug suspension to Blank ball core surface, get medicine ball core;
[0108] (2) Isolation gown...
Embodiment 3
[0115] Drugs and preparatory excipients
[0116] (1) drug layer
[0117] Blank ball core 80.0g
[0118] Lansoprazole 20.0g
[0120] Poloxamer 188 4.0g
[0121] Crospovidone 2.5g
[0122] Polyvinylpyrrolidone 10.0g
[0123] Proper amount of ethanol
[0124] (2) isolation layer
[0125] Talc powder 3.0g
[0126] Titanium dioxide 9.0g
[0127] Polyvinylpyrrolidone 10.0g
[0128] Anhydrous ethanol amount
[0129] (3) Enteric coating layer
[0130] Udrake L100-55 60.0g
[0131] Triethyl citrate 6.0g
[0132] Talc powder 15.0g
[0133] Anhydrous ethanol amount
[0134] Preparation:
[0135] (1) Drug layer preparation
[0136] Get an appropriate amount of ethanol, slowly add magnesia, polyvinylpyrrolidone, crospovidone and poloxamer that are sieved and mixed, stir evenly, add the lansoprazole crude drug to obtain a drug suspension, and spray the drug suspension to Blank ball core surface, get medicine ball core;
[0137] (2) Isolation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com