Catalyst for low-temperature water-gas-shift reaction under hydrogenous reformed gas and preparation method thereof
A technology for converting catalysts and water gas, applied in catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of reducing gold content, catalytic activity reduction, high gold content, etc. , to achieve the effect of increasing the contact interface concentration, reducing the catalytic activity, and improving the catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
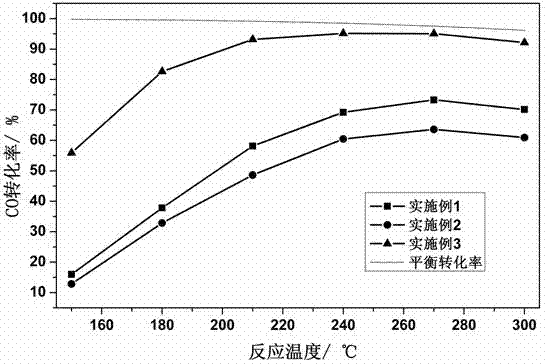
Embodiment 1
[0025] Take 60 mL of 0.4 mol / L Zr(NO 3 ) 4 ·5H 2 O aqueous solution was put into a 100 mL high-pressure polytetrafluoroethylene lining, and then the lining was put into a stainless steel outer lining, and after tightening, it was placed in a blast drying oven. Heating at 130 °C for 24 h. After the hydrothermal kettle is lowered to room temperature, pour out the precipitate in the lining, add a few drops of 25% concentrated ammonia water to the precipitate, adjust the pH of the system to 6, and then centrifugally wash the precipitate several times, and detect the absence of Cl by AgNO3. -1 until. The resulting precipitate was dried at 80 °C for 24 h, and then calcined in a muffle furnace at 200 °C for 6 h to obtain the desired ZrO 2 carrier. Take 2 g ZrO 2 Support preparation Au / ZrO 2 catalyst. First, 0.52 mL of 0.2 mol / L chloroauric acid solution was added dropwise to the ultrasonically dispersed ZrO 2 Carrier emulsion (ZrO 2 The carrier is dispersed in a certain amo...
Embodiment 2
[0027] Take 60 mL of 0.4 mol / L ZrO (NO 3 ) 2 2H 2 O aqueous solution was put into a 100 mL high-pressure polytetrafluoroethylene lining, and then the lining was put into a stainless steel outer lining, and after tightening, it was placed in a blast drying oven. Heat at 190°C for 3 h. After the hydrothermal kettle is lowered to room temperature, pour out the precipitate in the inner lining, add a few drops of 25% concentrated ammonia water to the precipitate, adjust the pH of the system to 10, then centrifugally wash the precipitate several times, and detect the absence of Cl by AgNO3 -1 until. The resulting precipitate was dried at 150 °C for 5 h, and then calcined in a muffle furnace at 550 °C for 2 h to obtain the desired ZrO 2 carrier. Take 2 g ZrO2 Support preparation Au / ZrO 2 catalyst. First, 0.52 mL of 0.2 mol / L chloroauric acid solution was added dropwise to the ultrasonically dispersed ZrO 2 Carrier emulsion (ZrO 2 The carrier is dispersed in a certain amount ...
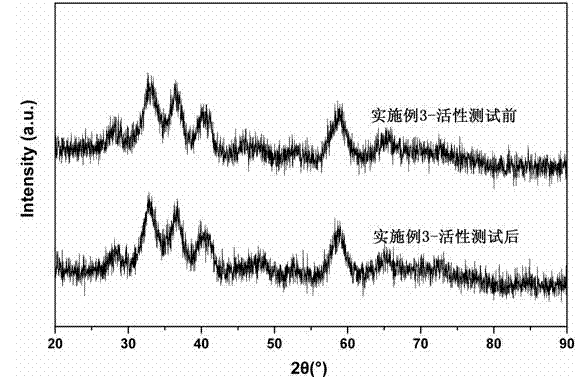
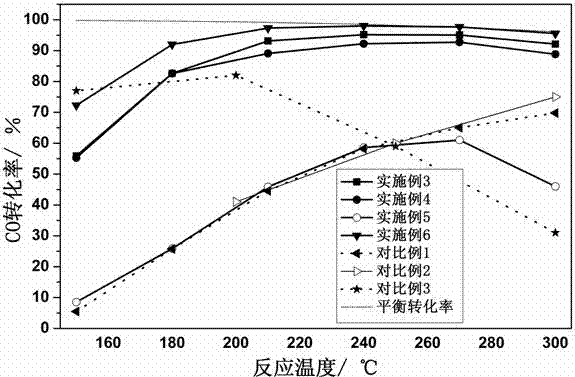
Embodiment 3
[0029] Take 60 mL of 0.4 mol / L ZrOCl 2 ·8H 2 O aqueous solution was put into a 100 mL high-pressure polytetrafluoroethylene lining, and then the lining was put into a stainless steel outer lining, and after tightening, it was placed in a blast drying oven. Heating at 150 °C for 6 h. After the hydrothermal kettle is lowered to room temperature, pour out the precipitate in the lining, add a few drops of 25% concentrated ammonia water to the precipitate, adjust the pH of the system to 8.8, and then centrifugally wash the precipitate for several times, and detect the absence of Cl by AgNO3 -1 until. The resulting precipitate was dried at 120 °C for 8 h, and then calcined in a muffle furnace at 350 °C for 4 h to obtain the desired ZrO 2 carrier. Take 2 g ZrO 2 Support preparation Au / ZrO 2 catalyst. First, 0.52 mL of 0.2 mol / L chloroauric acid solution was added dropwise to the ultrasonically dispersed ZrO 2 Carrier emulsion (ZrO 2 The carrier is dispersed in a certain amou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com