Clinical assay reagent, kit and method
A technology of clinical testing and kits, applied in the field of eliminating ascorbic acid interference, can solve the problems of ascorbic acid interference, affecting the reliability of clinical results, poor anti-interference ability of ascorbic acid, etc., and achieve the effect of increasing reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
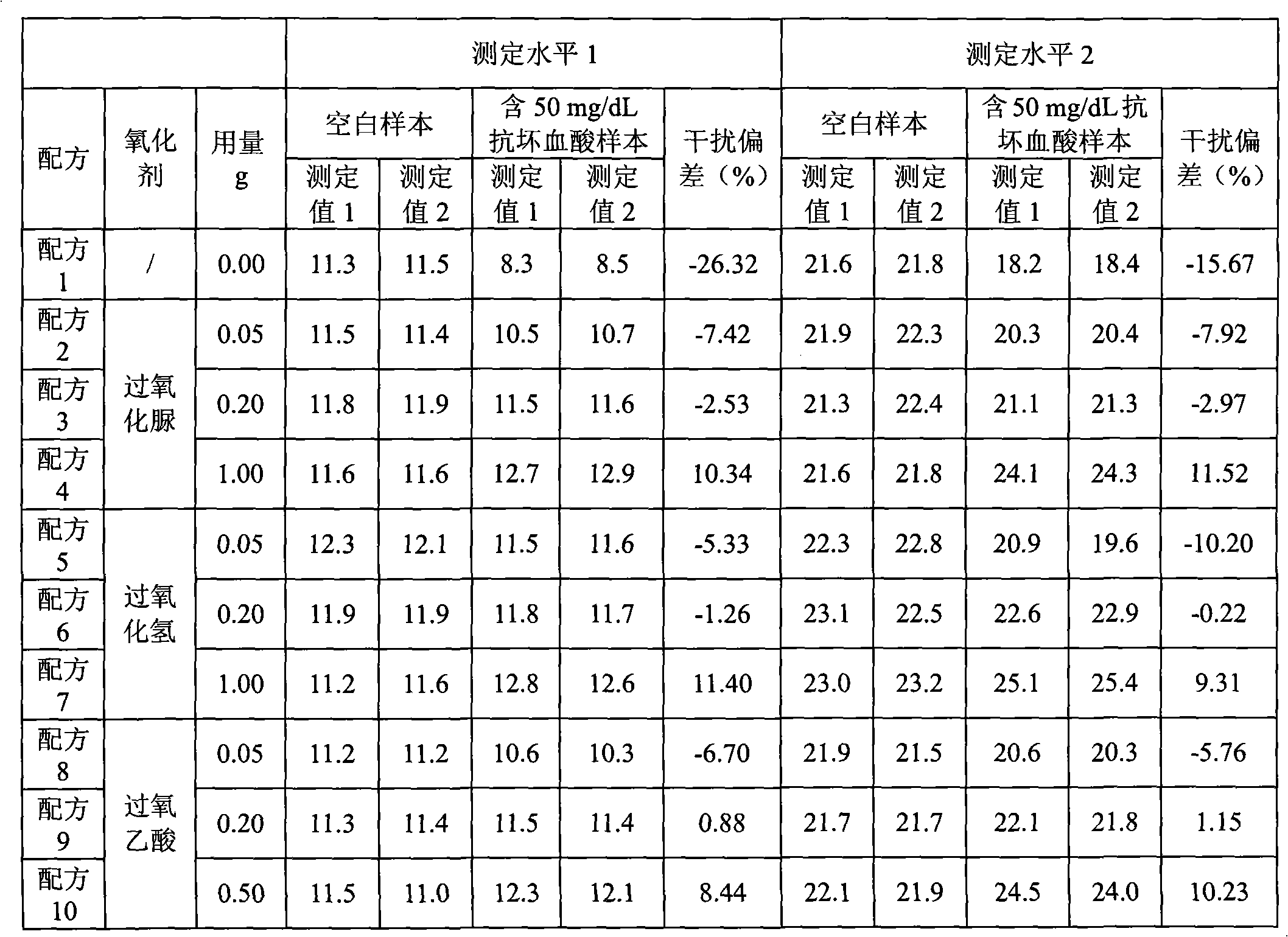
[0050] Elimination of ascorbic acid interference test of embodiment 1 peroxide
[0051] Formula Table
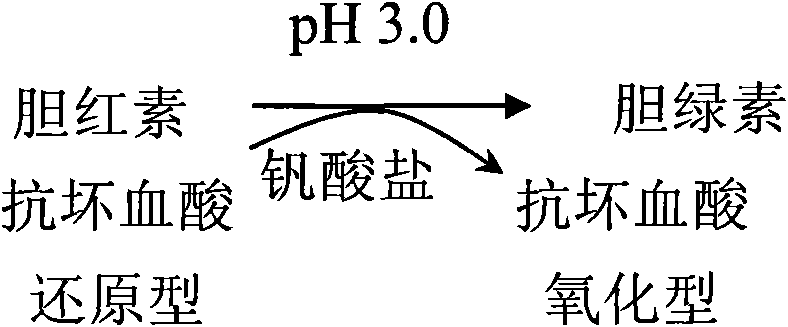
[0052] Reagent 1 (pH 3.0)
[0053] Citric acid 25.0g
[0054] Hydroxylamine Hydrochloride 3.0g
[0055] Thiourea 1.0g
[0056] Surfactant 1.0g
[0057] The specific dosage of carbamide peroxide (or hydrogen peroxide, or peracetic acid) is shown in Table 1
[0058] h 2 O to 1.0L
[0059] Reagent 2 (pH 7.0)
[0060] Sodium dihydrogen phosphate 1.0g
[0061] Sodium metavanadate 0.5g
[0062] h 2 O to 1.0L
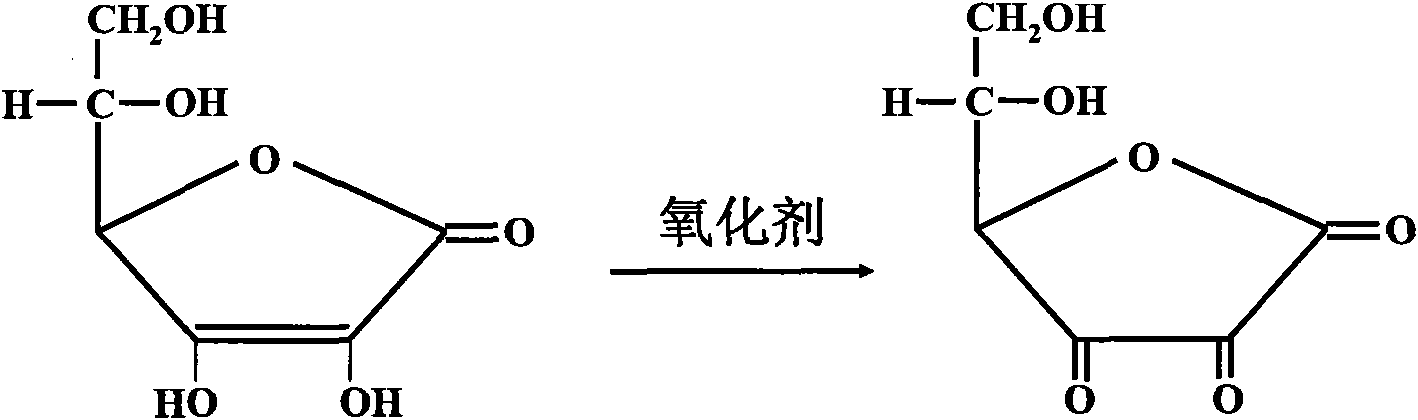
[0063] The method for determining direct bilirubin in a sample by using the embodiment of the present invention is as follows: add reagent 1 to the sample (calibration tube as a sample) and mix well, and read the absorbance A1 after incubating at 37°C for 3 to 5 minutes; add reagent 2, mix well , read the absorbance A2 after reacting at 37°C for 5 minutes; according to the formula: direct bilirubin content = measured absorbance (A2-A1) × calibration solution / ca...
Embodiment 2
[0069] Elimination of ascorbic acid interference test of embodiment 2 persulfate
[0070] Formula Table
[0071] Reagent 1 (pH 3.0)
[0072] Citric acid 20.4g
[0073] Hydroxylamine Hydrochloride 1.5g
[0074]Thiourea 0.8g
[0075] Surfactant 1.5g
[0076] The specific dosage of potassium persulfate (or sodium persulfate) is shown in Table 2
[0077] h 2 O to 1.0L
[0078] Reagent 2 (pH 7.0)
[0079] Sodium dihydrogen phosphate 1.0g
[0080] Sodium metavanadate 0.5g
[0081] h 2 O to 1.0L
[0082] Take by weighing the material in the formula table, take by weighing persulfate wherein by the amount in table 2, by the preparation method among the embodiment 1, prepare the test kit (prescription 12-17) that contains different kinds and concentration persulfate respectively , Formulation 11 is a persulfate-free kit. The sample and detection method for detection are the same as in Example 1. The results are shown in Table 2. The results show that when using the kit c...
Embodiment 3
[0085] Elimination of ascorbic acid interference test of embodiment 3 sulfate
[0086] Formula Table
[0087] Reagent 1 (pH 3.0)
[0088] Citric acid 25.0g
[0089] Hydroxylamine Hydrochloride 1.5g
[0090] Thiourea 1.2g
[0091] Surfactant 3.0g
[0092] The specific dosage of sodium sulfite (or sodium bisulfite) is shown in Table 3
[0093] h 2 O to 1.0L
[0094] Reagent 2 (pH 7.0)
[0095] Sodium dihydrogen phosphate 1.0g
[0096] Sodium metavanadate 0.5g
[0097] h 2 O to 1.0L
[0098] Take by weighing the material in the formula table, take by weighing the sulfite wherein in the amount in table 3, by the preparation method in the embodiment 1, prepare the test kit (prescription 19-24) that contains different kinds and concentrations of sulfite respectively , Formula 18 is a sulfite-free kit. The sample and detection method for detection are the same as in Example 1. The results are shown in Table 3. The results show that when using the kit containing the sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com