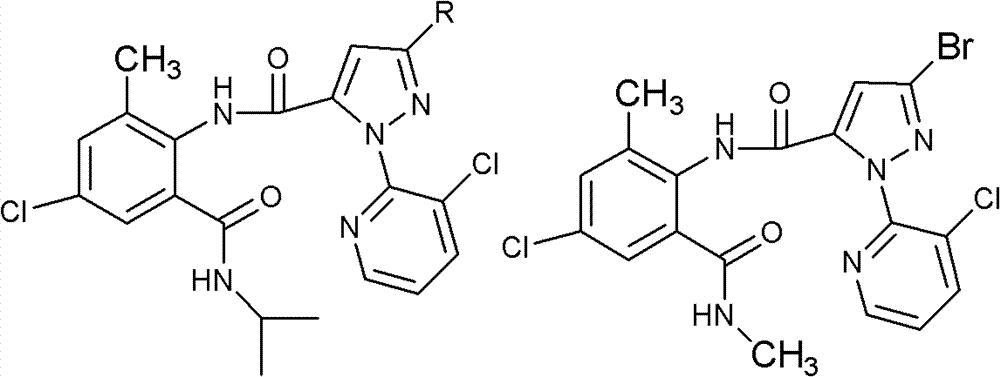
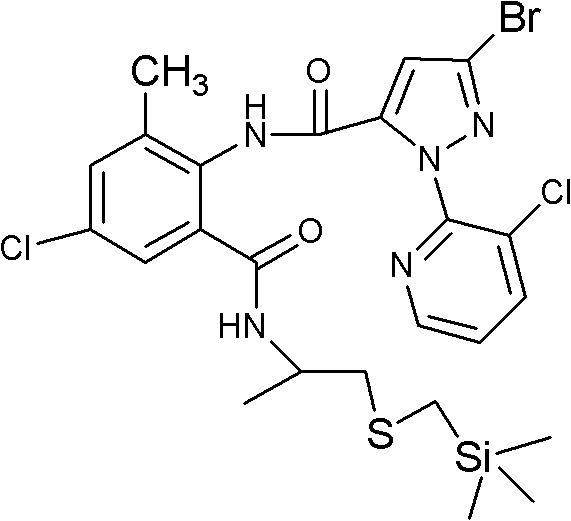
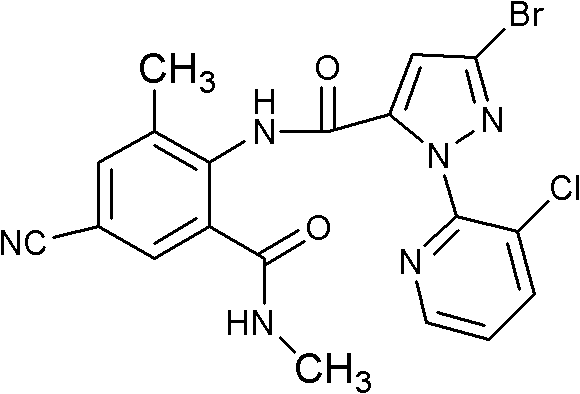
Fluoro methoxylpyrazole-containing o-formylaminobenzamide compound, synthesis method and application thereof
A technology of o-formamidobenzamide and monofluoromethoxypyrazole, applied in the field of o-formamidobenzamide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1N-[2-(tert-butylcarbamoyl)-4-chloro-6-methyl-phenyl]-1-(3-chloro-2-pyridyl)-3-fluoromethoxy- Synthesis of 1H-pyrazole-5-carboxamide (compound 8)
[0139] Step 1: Synthesis of 3-fluoromethoxy-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid
[0140] In a 500ml three-neck round bottom flask, add 1-(3-chloro-2-pyridyl)-3-hydroxyl-1H-pyrazole-5-carboxylic acid ethyl ester (13.35g, 0.05mol), 300ml acetonitrile, solid carbonic acid Potassium (8.28g, 0.06mol), bromofluoromethane (8.48g, 0.075mol), then heated to reflux, reacted until the raw materials completely disappeared, cooled to room temperature, filtered, the filter cake was rinsed with acetonitrile 2*50ml, and the filtrate was concentrated Add 200ml of methanol to dissolve it, then slowly add 50ml of aqueous solution containing sodium hydroxide (2.4g, 0.06mol) dropwise at room temperature, stir at room temperature for about 30min, the reaction is complete, distill off the solvent, add water, and diethyl e...
Embodiment 2
[0145] Example 2N-[2-(methylcarbamoyl)-4-chloro-6-methyl-phenyl]-1-(3-chloro-2-pyridyl)-3-fluoromethoxy-1H- Synthesis of Pyrazole-5-Carboxamide (Compound 3)
[0146] In a 50ml single-necked round bottom flask, add 6-chloro-2-[3-fluoromethoxy-1-(3-chloro-2-pyridyl)-1H-5-pyrazolyl]-8-methyl Add methylamine hydrochloride (0.19g, 0.00286mol) and anhydrous sodium acetate (0.24g, 0.00286mol), after stirring overnight at room temperature, the reaction was complete, the THF was evaporated under reduced pressure, water was added to the residue, extracted with ethyl acetate, and the organic phase was dried with anhydrous sodium sulfate. Filtration, concentration to obtain a crude product, and recrystallization with ethanol to obtain 0.87 g of a white solid, which is N-[2-(methylcarbamoyl)-4-chloro-6-methyl-phenyl]-1-(3-chloro -2-pyridyl)-3-fluoromethoxy-1H-pyrazole-5-carboxamide, melting point 236.0-237.4°C, yield 82.7%; 1 HNMR (400MHz, DMSO-d 6 )δ2.18(s, 3H), 2.93-2.94(d, 2H), 5.83...
Embodiment 3
[0147] Example 3N-[2-(ethylcarbamoyl)-4-cyano-6-methyl-phenyl]-1-(3-chloro-2-pyridyl)-3-fluoromethoxy-1H -Synthesis of pyrazole-5-carboxamide (compound 33)
[0148] The first step: 6-cyano-2-[3-fluoromethoxy-1-(3-chloro-2-pyridyl)-1H-5-pyrazolyl]-8-methyl-4H-[ d] Synthesis of [1,3] benzoxazin-4-one
[0149]In a 250ml three-neck round-bottomed flask, 3-fluoromethoxy-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid (5g, 0.0185mol) (Example 1-Synthesis No. One-step product), 2-amino-5-cyano-3-methylbenzoic acid (3.24g, 0.0185mol), acetonitrile 150ml, pyridine 15ml, stirring to dissolve all the solids, cooling the system, at -5°~- Add methanesulfonyl chloride (4ml, 5.98g, 0.052mol) / 20ml of acetonitrile solution dropwise at 10°. After the dropwise addition, keep it at -5°~-10° for 1h, then naturally rise to room temperature for 3h, after the reaction is complete , add 30ml of water, stir for 30min, filter, filter the cake successively with 2:1 acetonitrile / water (2*40ml), ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com