Improved production process of Shengmai injection
A technology for Shengmai injection and production process, applied in the field of Shengmai injection and production process of Shengmai injection, can solve problems such as side effects of Shengmai injection and affect the therapeutic effect, and achieve the safety and reduction of drug use. Pain response, the effect of eliminating vascular irritation and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
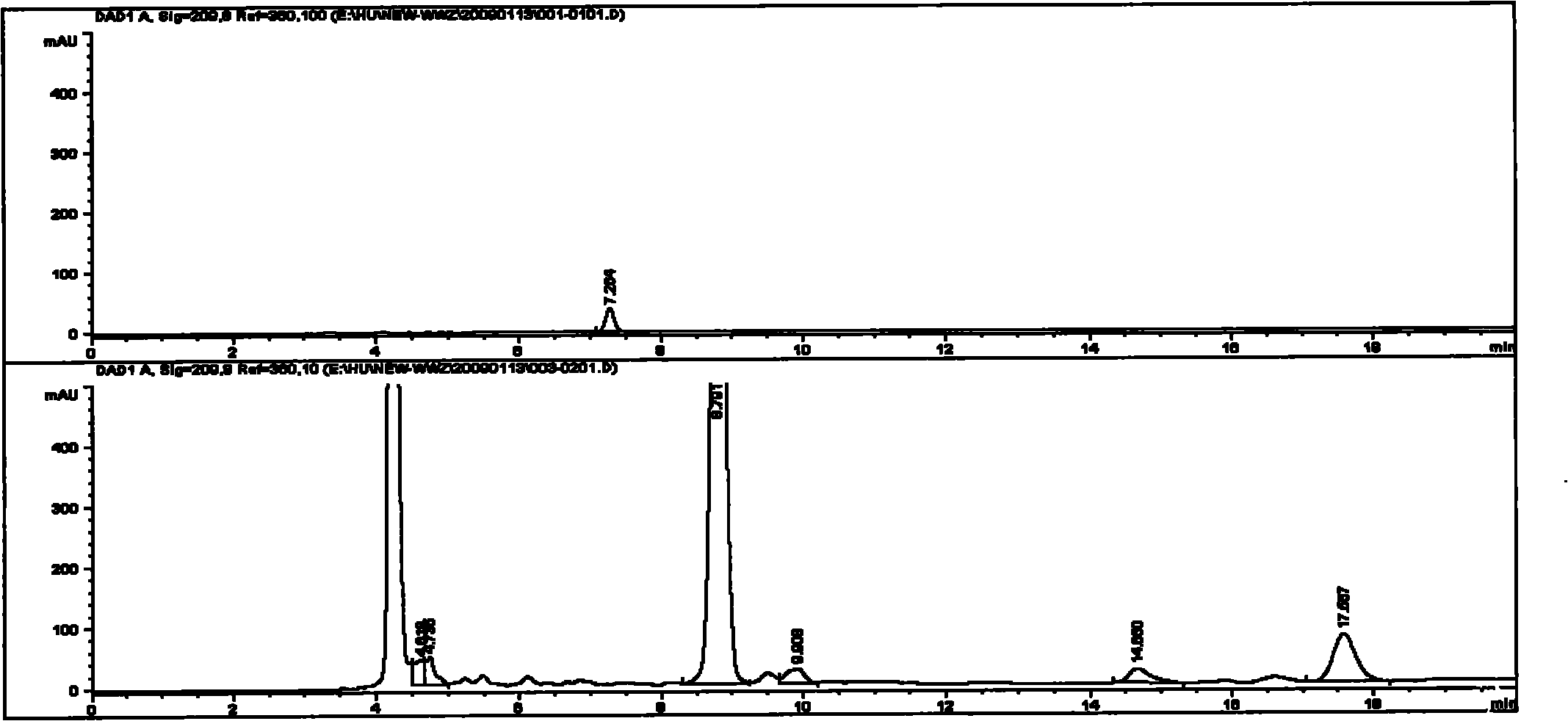
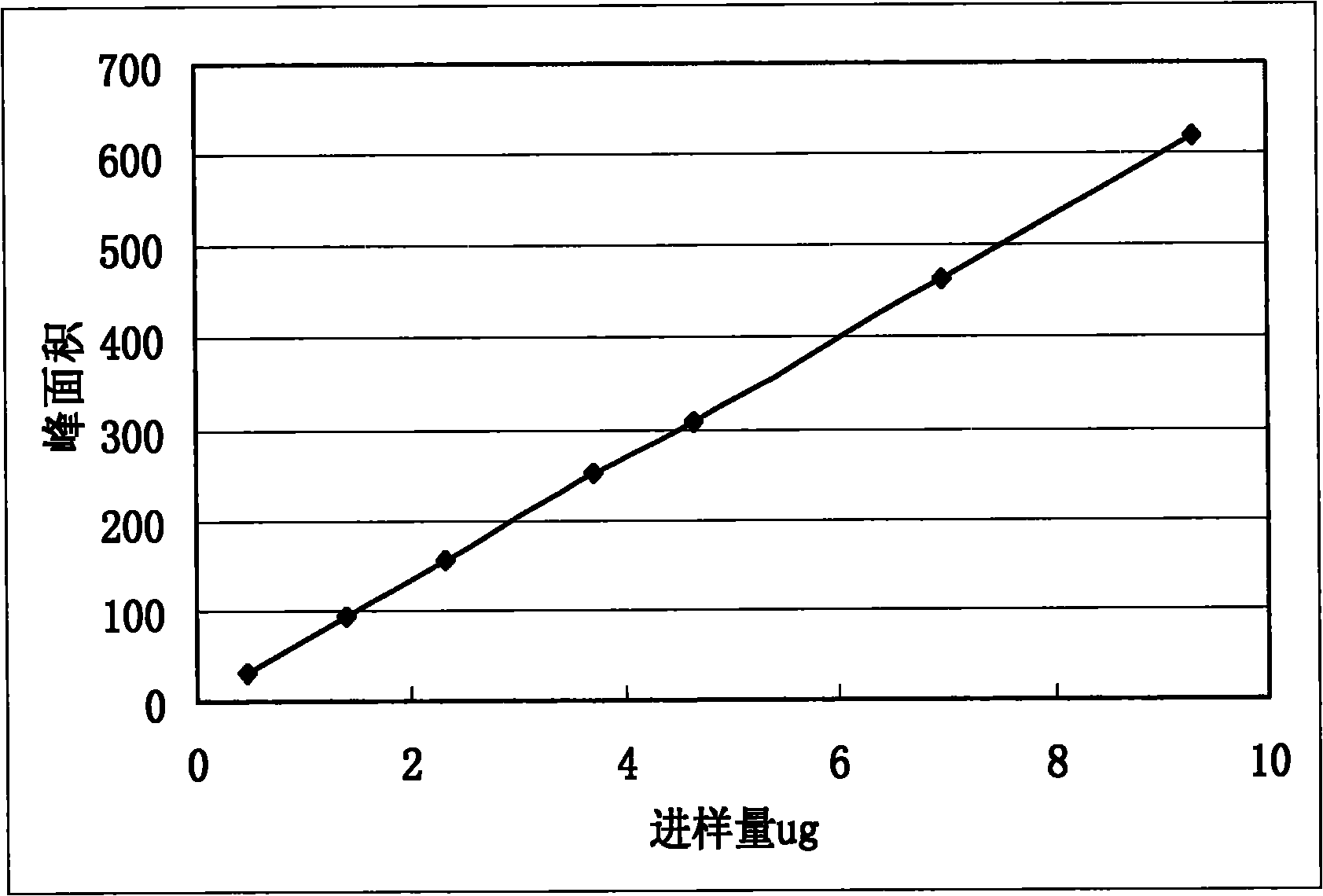
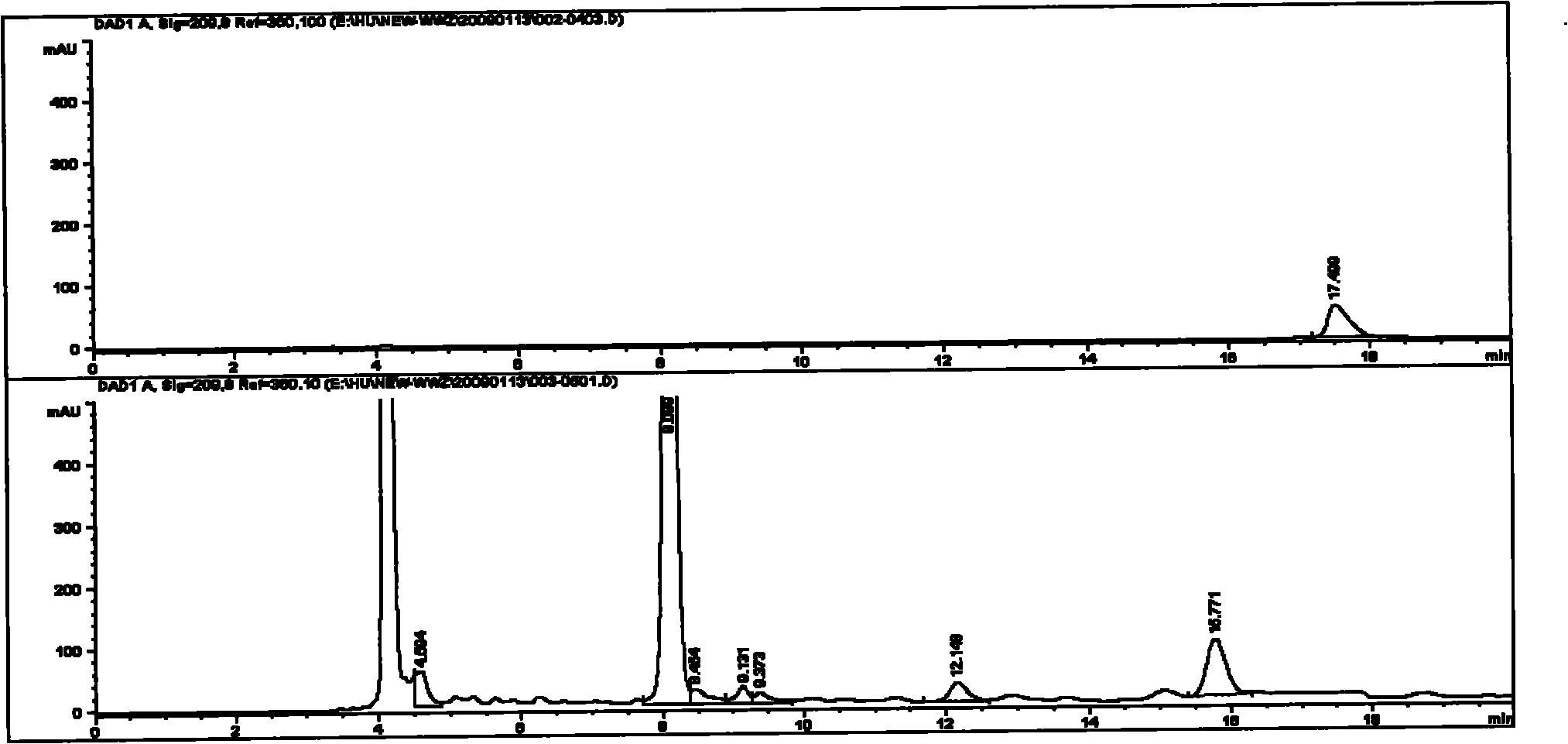
Image
Examples
Embodiment 1
[0020] Shengmai Injection was originally published in Volume 15 of the Ministry of Health Drug Standards for Traditional Chinese Medicine Preparations (WS 3 -B-2865-98) Shengmai injection prescription and process:
[0021] [Recipe] Red Ginseng 100g; Ophiopogon japonicus 312g; Schisandra 156g
[0022] [Production process] For the above three flavors, take red ginseng coarse grains (or thin slices), add ethanol to reflux extraction 5 times, each time for 2 hours, combine the extracts, filter, refrigerate the filtrate, recover ethanol, add NaOH to adjust the pH to 6.5, and steam to No alcohol smell, refrigerate, filter, and the filtrate is used for liquid preparation; take coarse powder of schisandra chinensis, soak in cold water, collect the distillate by steam distillation, and use it for liquid preparation; decoct the dregs of the medicine twice, combine the decoction, and filter , the filtrate is concentrated to a thick paste, add ethanol until the alcohol content reaches 85...
Embodiment 2
[0026] Example 2 Improved production process of Shengmai Injection
[0027] [Recipe] Red Ginseng 100g; Ophiopogon japonicus 312g; Schisandra 156g
[0028][Method] For the above three flavors, take red ginseng coarse grains (or thin slices), add ethanol to reflux extraction 5 times, each time for 2 hours, combine the extracts, filter, refrigerate the filtrate, recover ethanol, add NaOH to adjust the pH to 6.5, and steam to No alcohol smell, refrigerate, filter, and the filtrate is used for liquid preparation; take the coarse powder of Schisandra chinensis, soak in cold water, collect the distillate of Schisandra chinensis by steam distillation, and use it for liquid preparation; add water to decoct the dregs twice, combine the decoction, filter After filtering, the filtrate is concentrated to a thick paste, adding ethanol until the alcohol content reaches 85%, refrigerating, filtering, adjusting the pH to 6.5-7 with 35% sodium hydroxide solution in the filtrate, refrigerating, ...
Embodiment 3
[0029] The assay method of malic acid in the Shengmai Injection produced by the improved production process of embodiment 3 and its medicinal materials and intermediates
[0030] 1. Instruments, reagents and samples
[0031] 1.1 Instrument
[0032] High performance liquid chromatography: Agilent 1100; autosampler: G1322A; column thermostat: G1316A; detector: DAD G1315B; ultrasonic cleaner: SK7200LH, Shanghai Kedao Ultrasonic Instrument Co., Ltd.
[0033] 1.2 Reagents and reagents
[0034] Citric acid reference substance: National Institute for the Control of Pharmaceutical and Biological Products; batch number: 111679-200401; for content determination.
[0035] L-malic acid reference substance: SIGMA Company; batch number: 01011AE; for content determination.
[0036] Methanol is chromatographically pure, water is double distilled water, and other reagents are analytically pure.
[0037] The medicinal material of Schisandra was provided by the material warehouse and sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com