Apitoxin liposome preparation and preparation method thereof
A liposome preparation and melittin technology, applied in the preparation of melittin liposome preparation, the field of melittin liposome preparation, can solve the problem that melittin vascular irritation can not be completely eliminated, and improve the bioavailability , prolong the cycle time, improve the effect of compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Take by weighing 1.2g soybean lecithin (purity is greater than 92% phosphatidylcholine), 150mg cholesterol is dissolved in 50ml ethanol, ultrasonic, dissolves; Under 50 ℃, 400rpm magnetic stirring conditions, above-mentioned solution is slowly injected into 90ml isotonic saline ( Containing 5% poloxamer 188, which is percentage by weight, the same below), the two are mixed and stirred for 2 hours until the ethanol is completely evaporated to dryness to obtain a liposome coarse suspension, which is homogenized under high pressure to reduce the particle size (5000psi, 3 times), that is. Finally, 60 mg of melittin was dissolved in 30 ml of isotonic saline (containing 5% poloxamer 188), then mixed with the above solution, and 12 g of lactose was added to dissolve in the mixed solution, after sterile filtration (membrane filter pore size 0.2 μm) , the subsequent filtrate is dispersed without sub-packing in vials, and freeze-dried.
[0069] After the freeze-dried liposomes w...
Embodiment 2
[0075] Weigh 1g of soybean lecithin (purity greater than 93%), and dissolve 200mg of cholesterol in dichloromethane, place the solution in a ground eggplant-shaped bottle, and evaporate it under reduced pressure on a rotary evaporator on a constant temperature water bath at 25°C at 100rpm. Organic solvent, so that phospholipids and cholesterol form a uniform film on the bottom of the bottle, and then put it in a vacuum desiccator to evacuate overnight, and set aside. In addition, 100 mg of melittin was dissolved in 200 ml of isotonic 5% glucose solution (containing 2% poloxamer 188), this solution was added to the above-mentioned eggplant-shaped bottle, and the membrane was washed by rotating at 37 ° C until milky white lipids were formed. Body suspension, homogenized under high pressure, reduced particle size (5000psi, 3 times), that is. Dissolve 20 g of trehalose in liposomes, filter aseptically (the pore size of the membrane filter is 0.2 μm), divide the filtrate into vials...
Embodiment 3
[0078] After the subsequent filtrate after aseptic filtration in Example 1 is subpackaged in vials, through repeated freezing and thawing (freezing temperature is -50 ℃, freezing time is 3h, melting temperature is 4 ℃, melting time is 30min, repeated freezing and thawing The number of times is 2 times), freeze-dried to obtain freeze-dried powder injection.
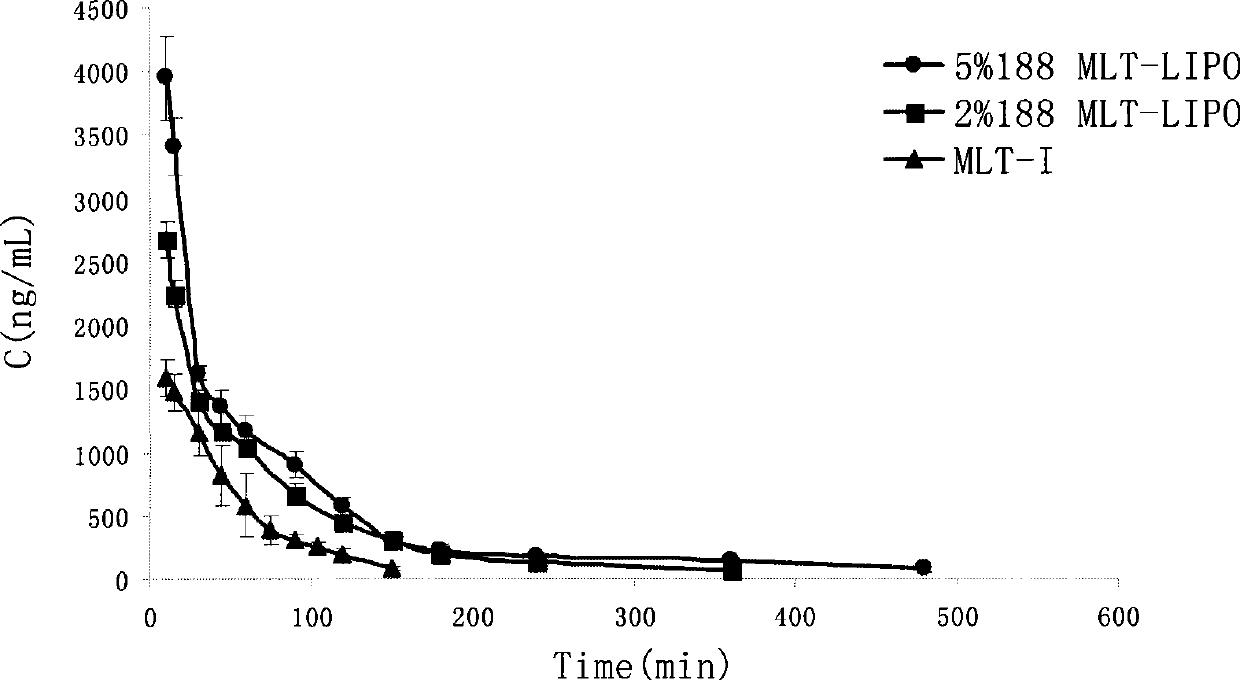
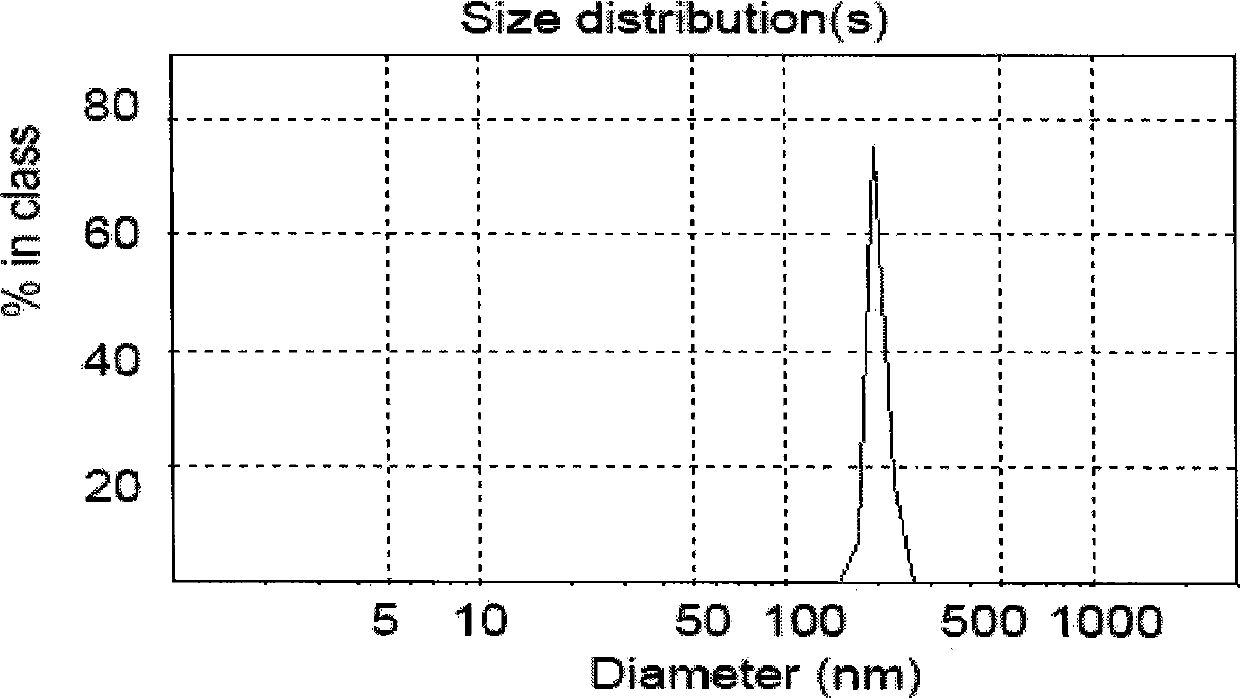
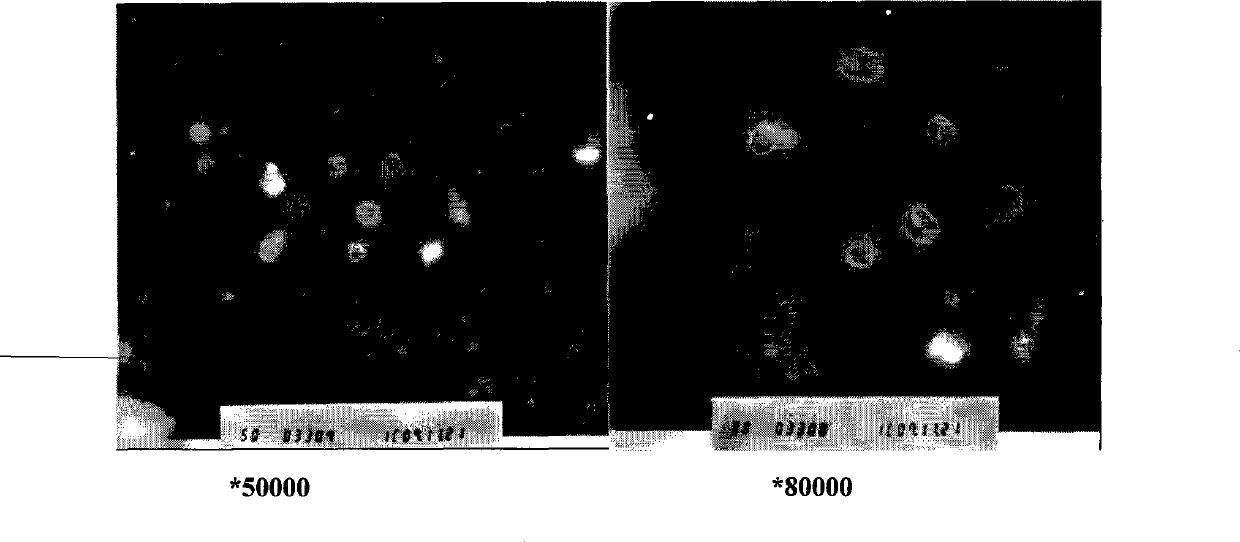
[0079] After the freeze-dried liposomes were stored at 4°C for 3 months, the melittin content was 95.9%. After adding an appropriate amount of injection-grade isotonic glucose solution, the liposome encapsulation efficiency was 96.8%, and the particle size was 214nm. The encapsulation efficiency remained basically unchanged after being diluted with 5% glucose solution or 0.9% normal saline for 8 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com