Preparation method of fibrauretine
A technology of palmetine and intermediates, which is applied in the field of compound preparation, can solve the problems of raw material acquisition restrictions, and achieve the effects of low synthesis cost, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
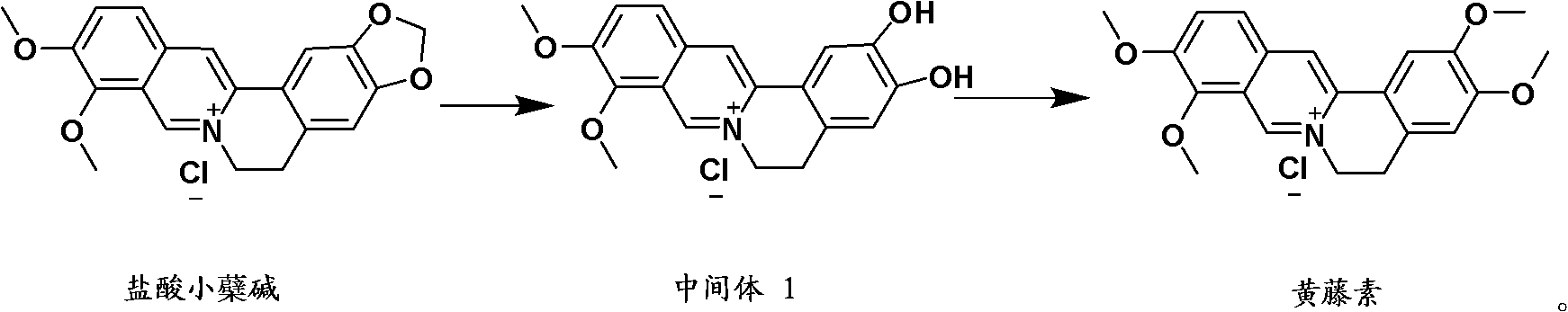
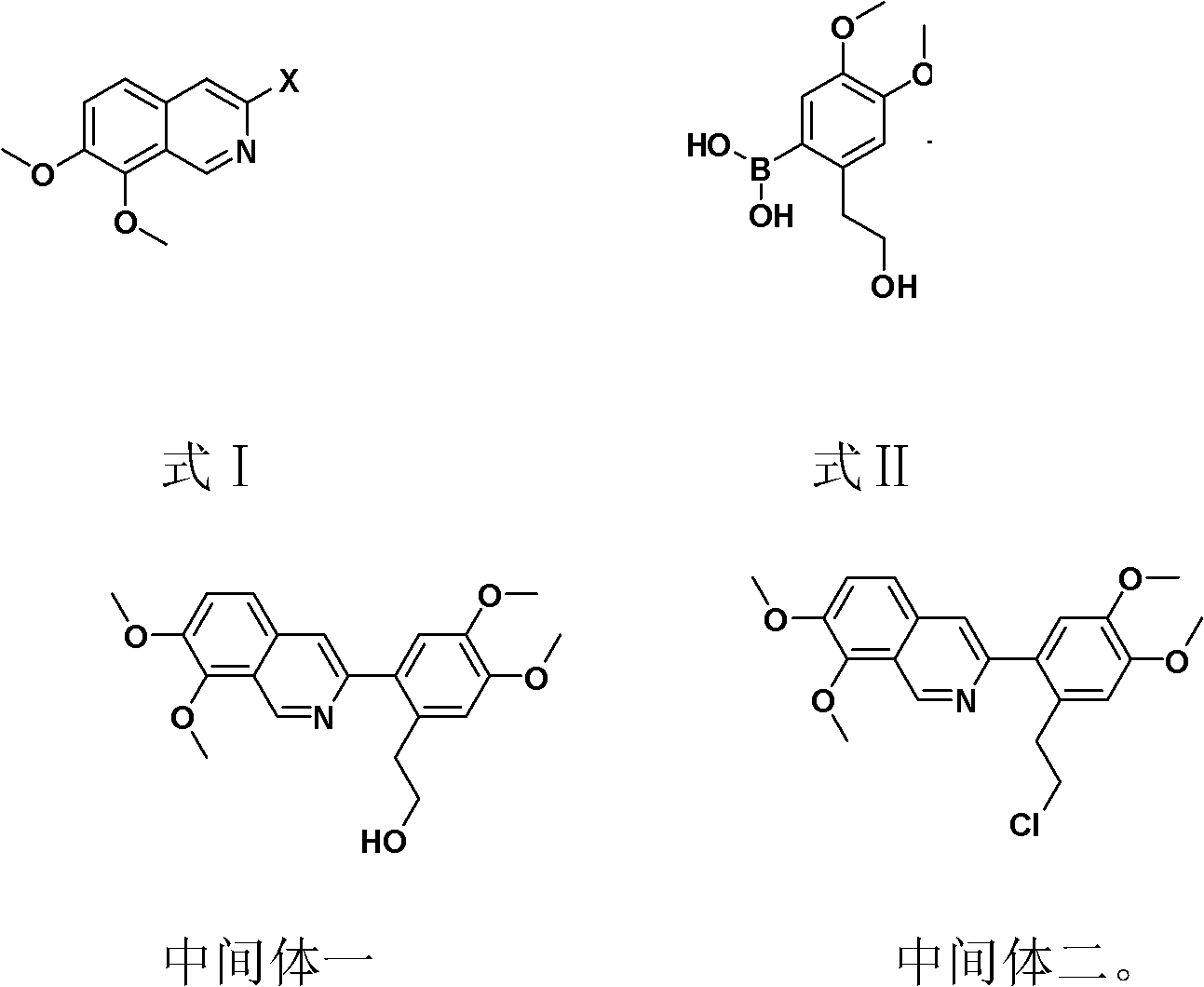
[0034] Synthesis of intermediate one:
[0035] Take a 5L dry three-necked bottle, add 268 grams of 3-bromo-7,8-dimethoxyisoquinoline, 238 grams of 2-(2-hydroxyethyl)-4,5-dimethoxyboronic acid in sequence , 200 grams of anhydrous potassium carbonate and 2.5L of anhydrous dioxane, nitrogen replacement three times under the condition of mechanical stirring, adding 10 grams of Pd (PPh 3 ) 4 , reflux reaction under nitrogen protection conditions, HPLC detection product content no longer rises, stop the reaction, cool to room temperature, and suction filter. The obtained filtrate recovered the solvent under reduced pressure, and the viscous material was dissolved in 2 L of dichloromethane, washed with 2 L of water, dried, and concentrated to obtain 315 g of solid, with a yield of 85.4%, which was directly used for the next reaction without purification.
[0036] Synthesis of Intermediate 2:
[0037] Take a 5L dry three-necked bottle, add 315g of intermediate one and 2L of anhydro...
Embodiment 2
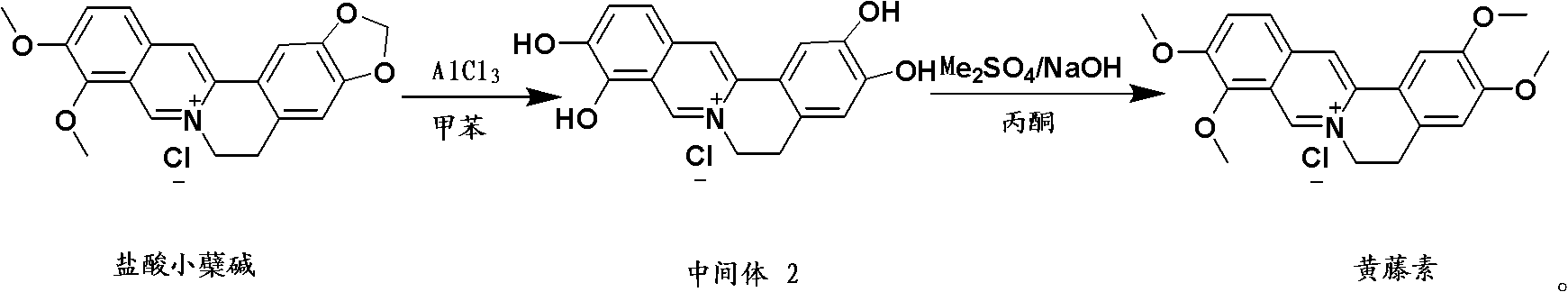
[0041] Synthesis of intermediate one:
[0042] Take a 5L dry three-necked bottle, add 200 grams of 3-bromo-7,8-dimethoxyisoquinoline and 178 grams of 2-(2-hydroxyethyl)-4,5-dimethoxyboronic acid in sequence , 120 grams of anhydrous Na 2 CO 3 and 1.5L of anhydrous toluene, nitrogen replacement three times under the condition of mechanical stirring, adding 15g of Pd(OAc) 2, reflux reaction under the protection of Ar, the HPLC detection product content no longer increased, stop the reaction, cool to 25 ° C, and suction filter. The obtained filtrate recovered the solvent under reduced pressure, the viscous material was dissolved with 1.5 L of dichloromethane, washed with 2 L of water, the obtained organic layer was dried over sodium sulfate, and concentrated to obtain 220 g of solid, with a yield of 80%, which was directly used for the next step reaction without purification.
[0043] Synthesis of Intermediate 2:
[0044] Take a 3L dry three-necked flask, add 220g of intermedi...
Embodiment 3
[0048] Synthesis of intermediate one:
[0049] Take a 3L dry three-necked bottle, add 150g 3-bromo-7,8-dimethoxyisoquinoline, 134g 2-(2-hydroxyethyl)-4,5-dimethoxyboronic acid, 65g Li 2 CO 3 and 1.5L of anhydrous acetone, replaced with nitrogen three times under the condition of mechanical stirring, and added 7g of PdCl 2 , reflux reaction under nitrogen protection conditions, HPLC detection product content no longer rises, stop the reaction, cool to room temperature, and suction filter. The obtained filtrate recovered the solvent under reduced pressure, and the viscous material was dissolved in 1 L of dichloromethane, washed with 1 L of water, dried, and concentrated to obtain 161 g of solid, with a yield of 78%, which was directly used for the next reaction without purification.
[0050] Synthesis of Intermediate 2:
[0051] Take a 2L dry three-necked flask, add 160g of intermediate one and 0.8L of anhydrous acetonitrile, add 120g of triphenylphosphine under nitrogen pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com