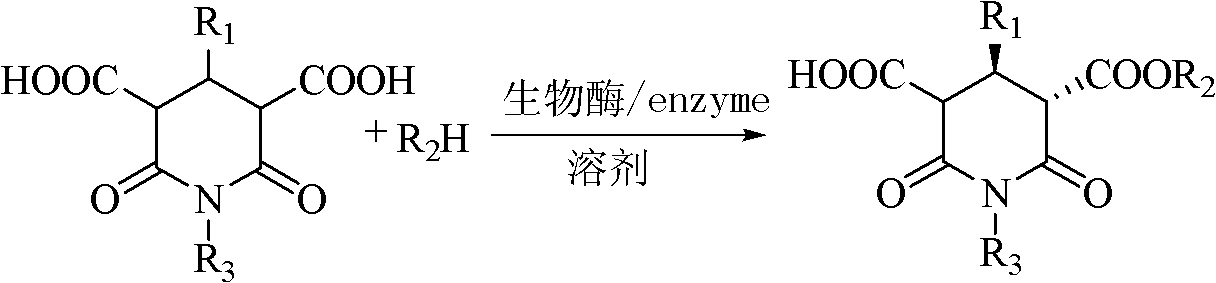
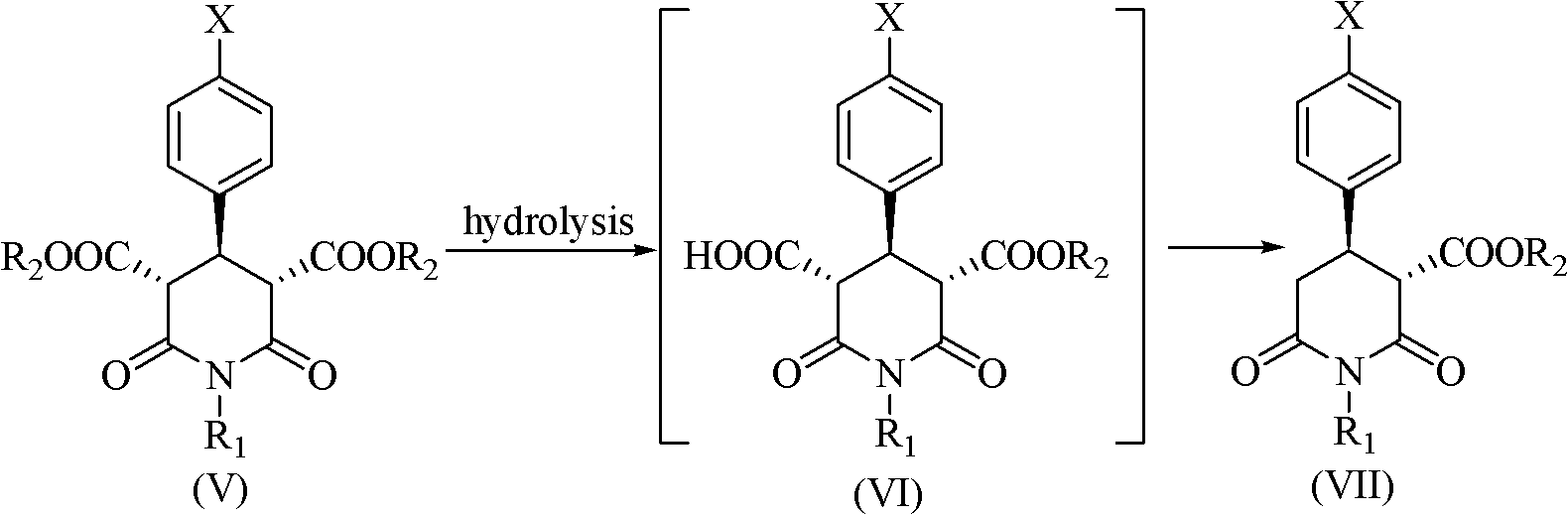
Method for preparing paroxetine intermediate by enzymatic selective hydrolysis in ionic liquid
An ionic liquid and selective technology, applied in the field of biocatalysis, can solve problems such as the unfavorable environmental protection of organic solvents, and achieve the effects of good chemical and thermal stability, low environmental pollution, and stereoselectivity maintenance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
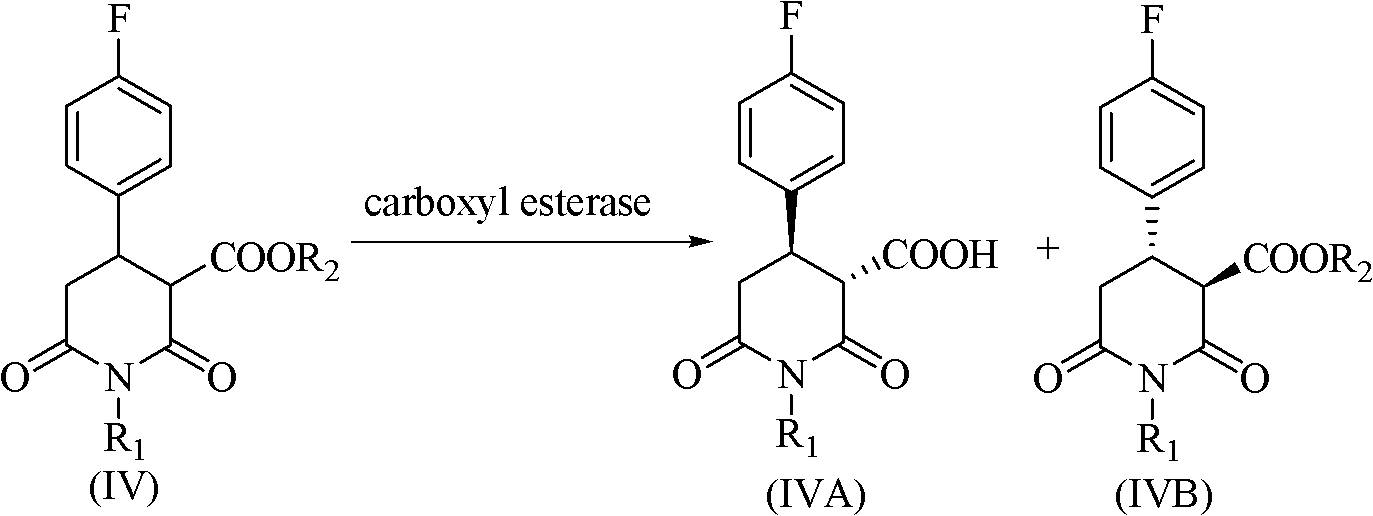
[0043] Get 0.5g of compound (IIA) in the reactor, add 5.0ml, pH7.0, 0.2M phosphate buffer, 1.5g of ionic liquid [BMIm][PF 6 ] and 10mg of Lipase A from Candida antarctica were reacted in a water bath shaker at 30°C and 200rpm for 24h, during which TLC tracked and detected the reaction process. After the reaction, the supernatant was obtained by centrifuging to remove the biological enzyme, and the supernatant was rotary evaporated to remove water and ethanol generated, then added ethyl acetate for extraction, and the raffinate recovered the ionic liquid, and the extract was added with a small amount of anhydrous Na 2 SO 4 After drying, the reaction conversion and product purity were analyzed by gas chromatography.
[0044] (4R,5S)-5-Ethyl carboxylate-4-(4-fluorophenyl)-1-R-2,6-dioxopiperidine-3-carboxylic acid, the conversion rate is 87%, and the purity is 79%.
Embodiment 2
[0046] Get 0.5g of compound (IIA) in the reactor, add 5.0ml, pH7.0, 0.2M phosphate buffer, 1.5g of ionic liquid [BMIm][PF 6 ] and 10mg of Lipase B from Candida antarctica were reacted in a water-bath shaker at 30°C and 200rpm for 18h, during which TLC tracked and detected the reaction process. After the reaction, the supernatant was obtained by centrifuging to remove the biological enzyme, and the supernatant was rotary evaporated to remove water and ethanol generated, then added ethyl acetate for extraction, and the raffinate recovered the ionic liquid, and the extract was added with a small amount of anhydrous Na 2 SO 4 After drying, the reaction conversion and product purity were analyzed by gas chromatography.
[0047] (4R,5S)-5-Ethyl carboxylate-4-(4-fluorophenyl)-1-R-2,6-dioxopiperidine-3-carboxylic acid, conversion rate 97%, purity 91%.
Embodiment 3
[0049] Get 0.5g of compound (IIA) in the reactor, add 5.0ml, pH7.0, 0.2M phosphate buffer, 1.5g of ionic liquid [BMIm][PF 6 ] and 15mg of Esterase from Porcine Liver were reacted in a water-bath shaker at 30°C and 200rpm for 24h, during which TLC tracked and detected the reaction process. After the reaction, the supernatant was obtained by centrifuging to remove the biological enzyme, and the supernatant was rotary evaporated to remove water and ethanol generated, then added ethyl acetate for extraction, and the raffinate recovered the ionic liquid, and the extract was added with a small amount of anhydrous Na 2 SO 4 After drying, the reaction conversion and product purity were analyzed by gas chromatography.
[0050] (4R,5S)-5-Ethyl carboxylate-4-(4-fluorophenyl)-1-R-2,6-dioxopiperidine-3-carboxylic acid, conversion rate 98%, purity 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com