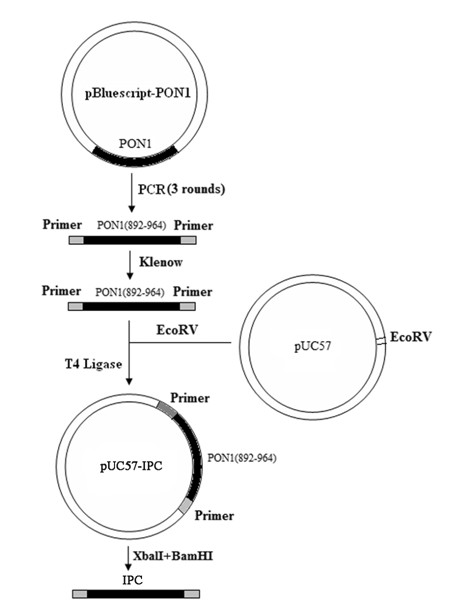
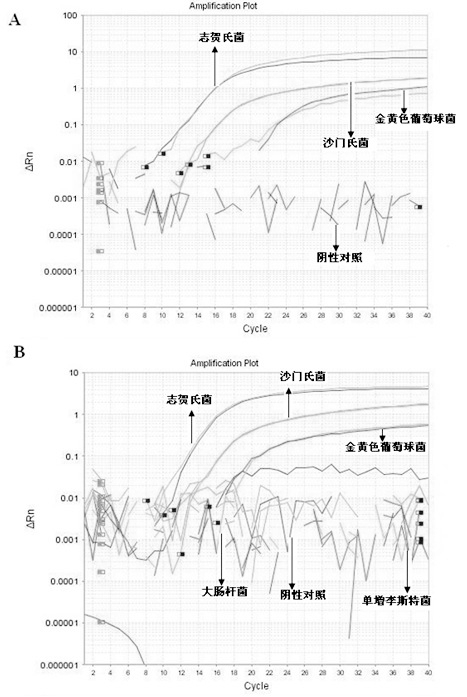
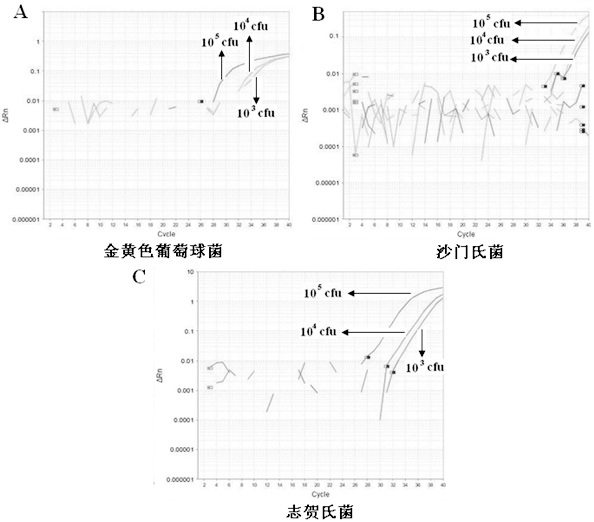
Multiple fluorescence quantitative PCR method for simultaneous and fast detecting three types of pathogenic bacteria in food
A multiple fluorescence quantitative and pathogenic bacteria technology, applied in the direction of microbial measurement/inspection, fluorescence/phosphorescence, biochemical equipment and methods, etc., can solve the problems of lack of positive internal reference, etc., achieve high throughput, good practicability, and operation convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] 1. Experimental materials, reagents and instruments
[0027] DNA microquantification kit, Invitrogen Company, USA; Taq enzyme and buffer, Takara Company, Japan; media were purchased from Beijing Luqiao; DNA molecular weight standards, lysozyme, proteinase K, etc. were purchased from Nanjing Shengxing; primers and double-labeled Taqman The probes were all synthesized by Shanghai Shenggong Company. Fast fluorescent quantitative PCR instrument, ABI 7500 Fast, American ABI Company; PCR instrument, American ABI Company; high-speed centrifuge, Sigma3-18K, American Sigma Company.
[0028] 2. Simultaneous rapid enrichment of Salmonella, Staphylococcus aureus and Shigella
[0029] Staphylococcus aureus CECT86T, Salmonella S34 and Shigella ATCC8700 each 1×10 1 At the same time, 1 mL of raw milk or 10% pork homogenate was added to prepare artificially contaminated food samples. Prepare the mixed culture medium of Gram-negative bacteria general GN medium and 7.5% NaCl broth vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com