Method for extracting electrolytic zinc from oxide material containing less than 20% of zinc by using waste acid in titanium white plant
A technology of material and zinc content, applied in the direction of improving process efficiency, can solve the problem of inability to produce electrolytic zinc with industrial-grade sulfuric acid, and achieve the effect of effective development and utilization and reduction of production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
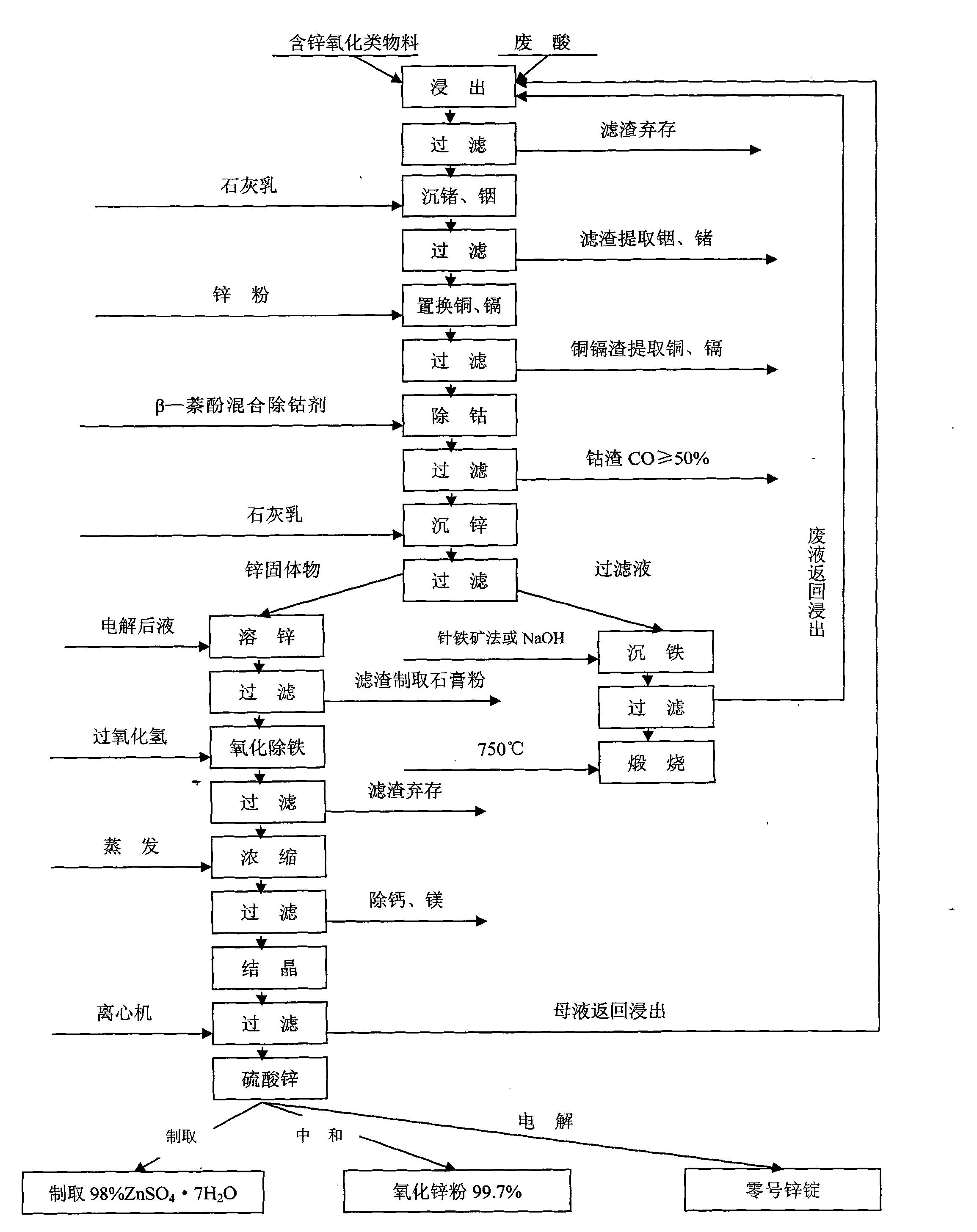
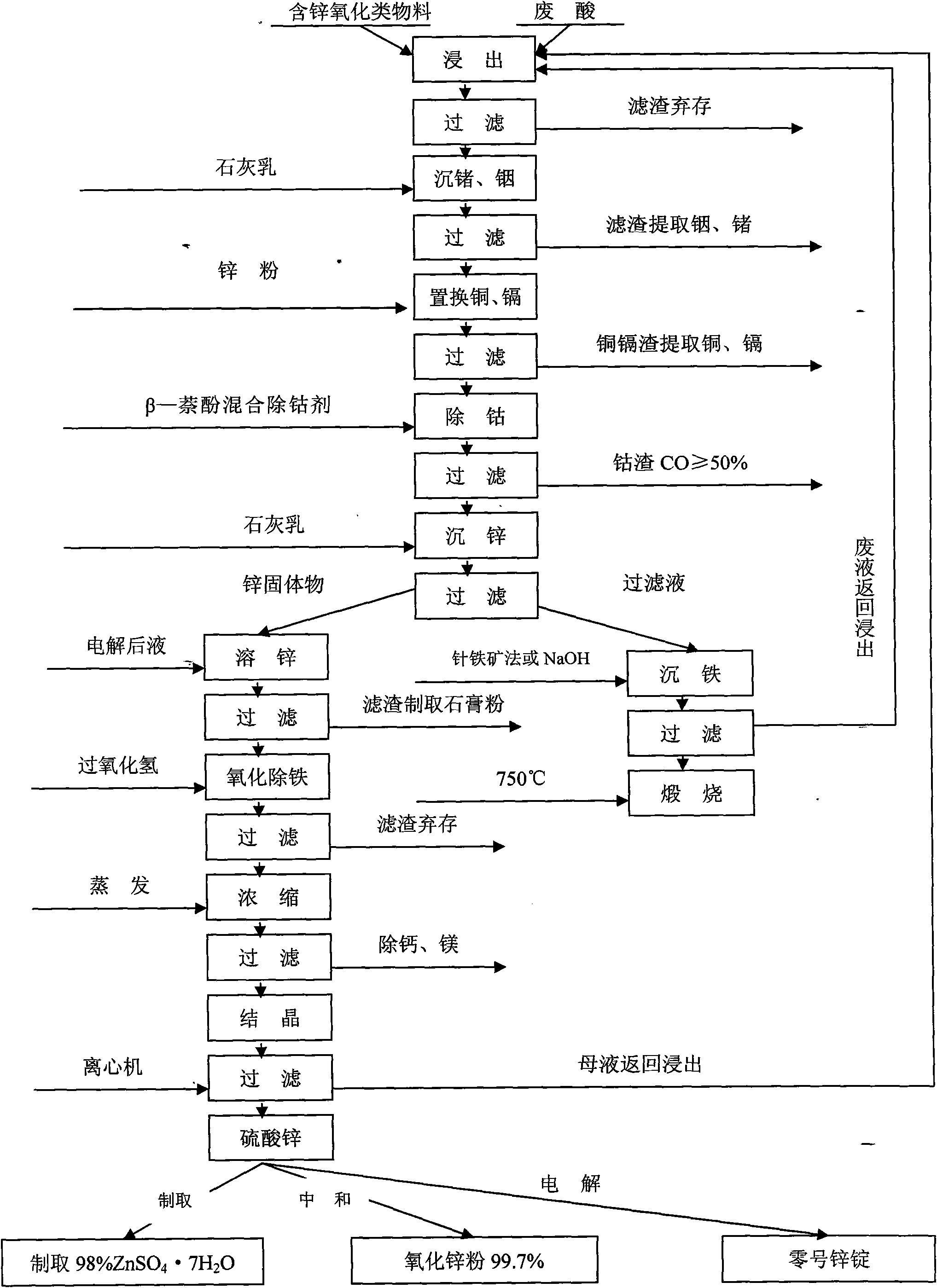
[0032] Use waste acid from sulfuric acid titanium dioxide factory (content of sulfuric acid is 18-22%) as leaching agent to leach zinc oxide, indium, germanium, copper, cadmium, iron, etc. in oxidized materials with zinc content below 20% metal substance.
[0033] At room temperature, stirring and leaching are carried out at a speed of 60-80 revolutions per minute. The stirring time is 2 to 3 hours, and the amount of waste acid used is 1:4. That is, 1 part of zinc-containing oxidized material, 4 parts of waste acid, and a solid-to-liquid ratio of 1:6, until the pH value of the leaching solution is stabilized below 1.0, and after an extension of 1 hour, the stirring can be stopped. The chemical reaction formula is:
[0034] ZnO+H 2 SO 4 →ZnSO 4 +H 2 o
[0035] In 2 o 3 +3H 2 SO 4 →In 2 (SO 4 ) 3 +3H 2 o
[0036] GeO+H 2 SO 4 →GeSO 4 +H 2 o
[0037] CdO+H 2 SO 4 →CdSO 4 +H 2 o
[0038] CuO+H 2 SO 4 →CuSO 4 +H 2 o
[0039]Iron mainly comes from wast...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com