Hypoallergenic hybrid proteins of major group 1 and 2 mite allergens for use in the treatment of allergies
An allergen-like, allergen-like technology, applied in the directions of allergen antigen components, gene therapy, fusion polypeptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] Example 1: Purification of natural allergens Der p 1 and Der p 2 from the body of mites
[0195] A mixture of lyophilized body and feces of D. dust mites (Laboratorios Leti, Madrid, Spain) was used as starting material in 10 volumes (p / v) supplemented with 1 mm PMSF (phenylmethanesulfonyl fluoride) in PBS (phosphate buffered). brine) was extracted with rapid stirring for 15 minutes at 4°C. It was then centrifuged at 3,800xg for 15 minutes at 4°C. The extraction supernatant was filtered through AP (Millipore) and 60% ammonium sulfate 361 g / l) was added slowly for 30 min. After stirring for 1 hour at 4°C, it was centrifuged at 17,000 xg for 15 minutes at 4°C.
[0196] · Purification of native Der p 1
[0197]The pellet obtained after centrifugation was resuspended in 2 ml of 20 mM Tris pH 8.0 and filtered through 0.22 μm. Molecular sieve chromatography was performed on a Superdex S20016 / 60 column (GE-Healthcare, Uppsala, Sweden) equilibrated with PBS and injected wi...
Embodiment 2
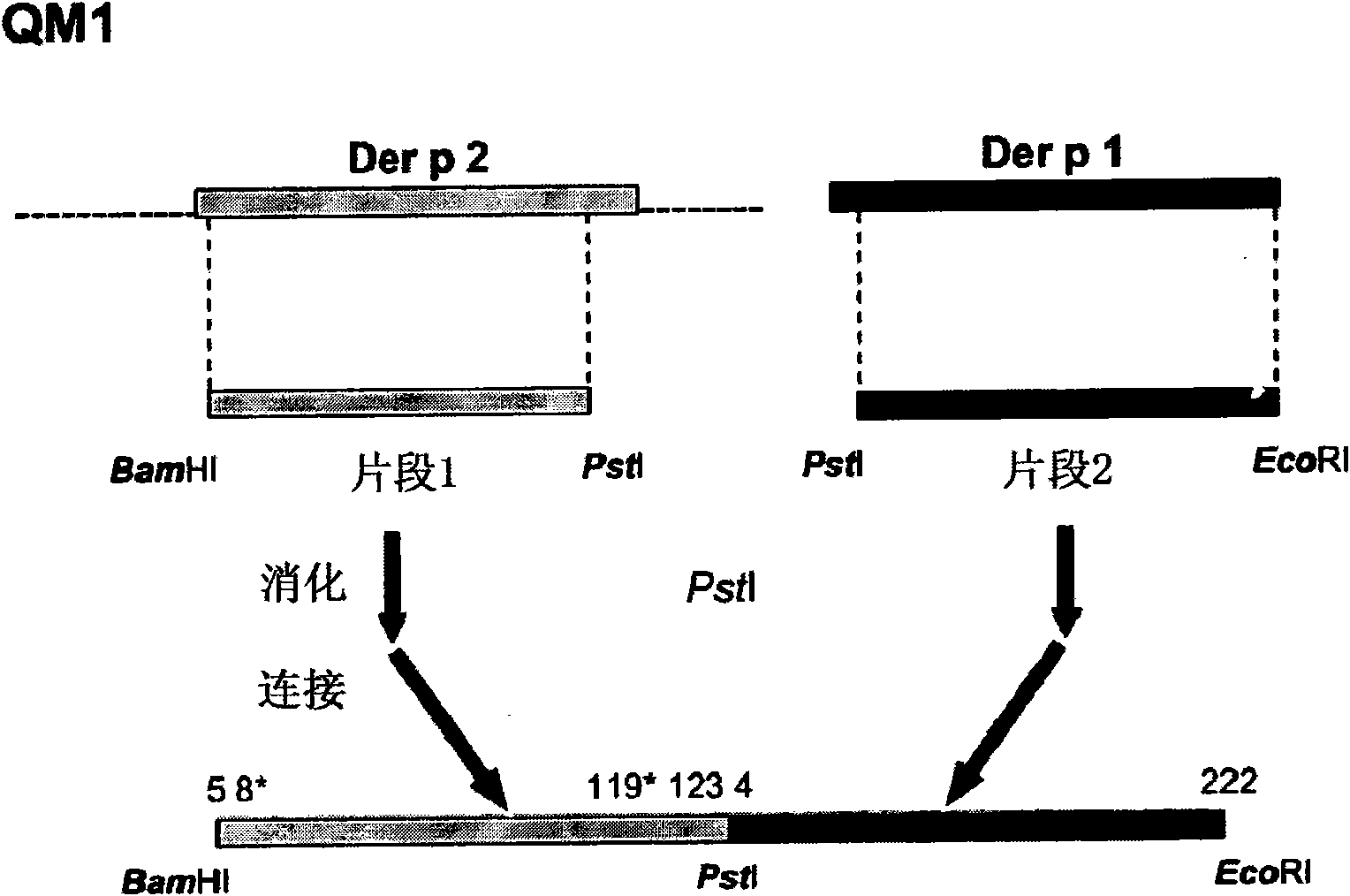
[0203] Example 2: Cloning of Der p 1 and Der p 2 allergens
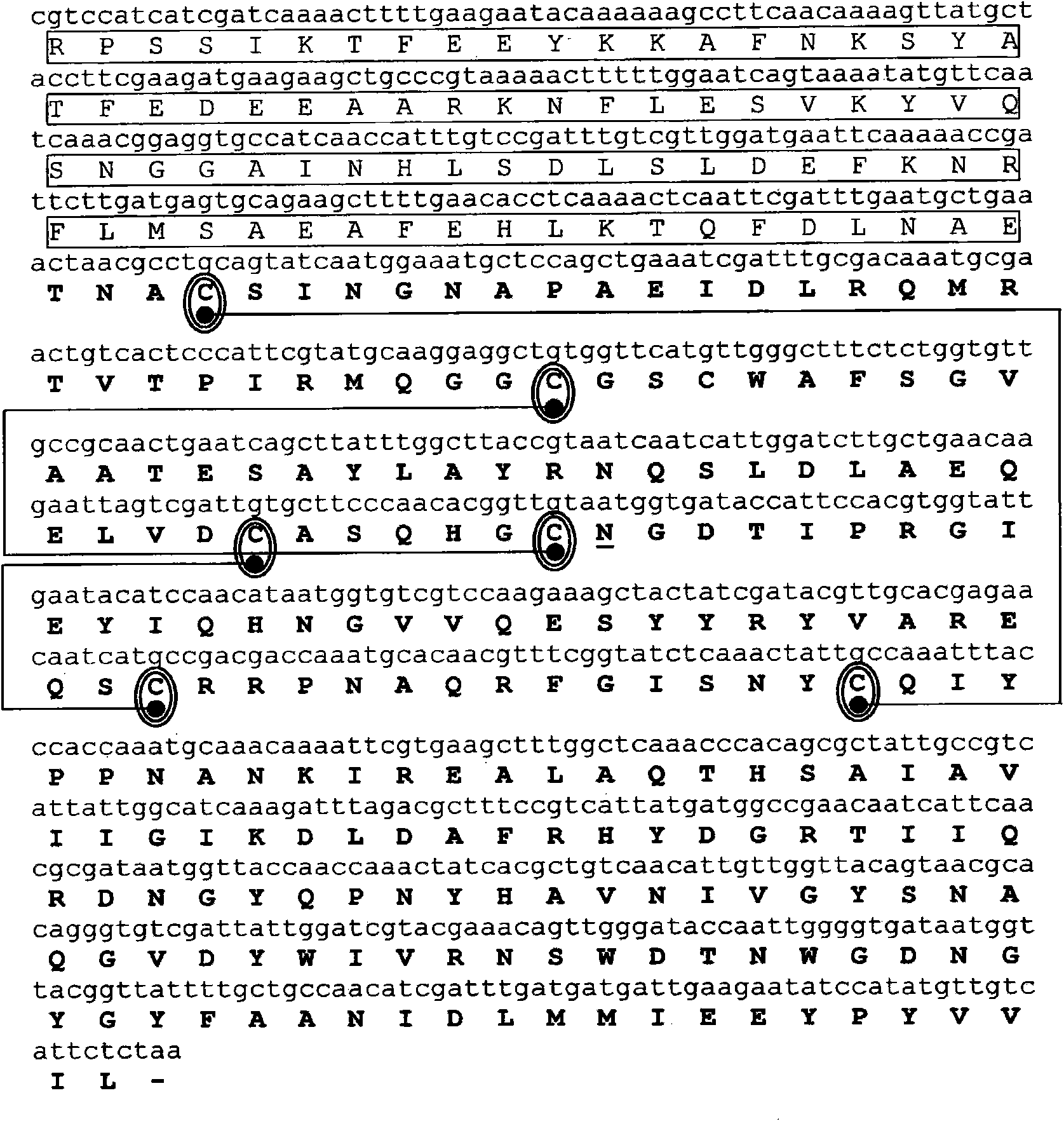
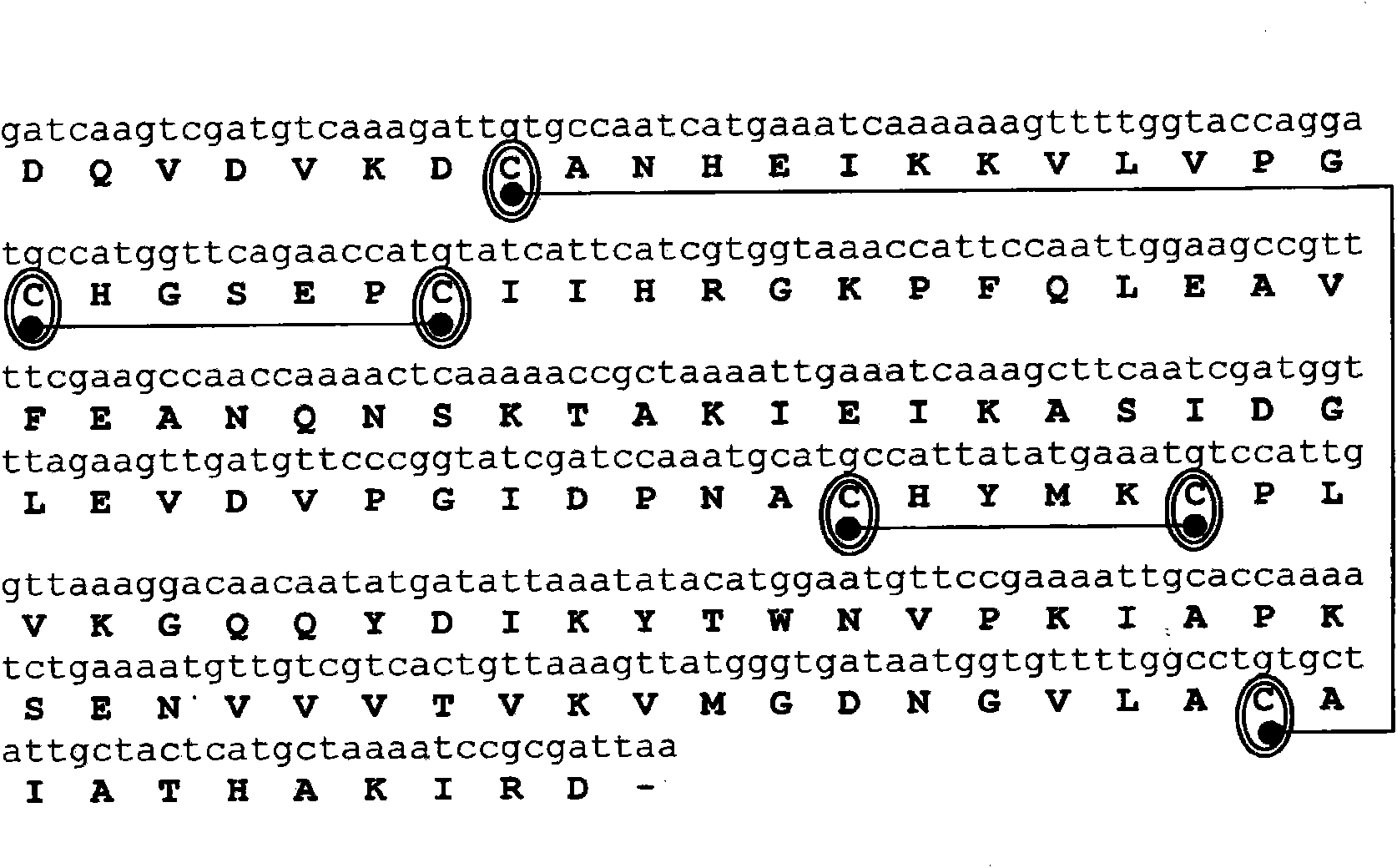
[0204] Complementary DNA (cDNA) encoding the allergens Der p 1 and Der p 2, in each case cloned by reverse transcription using mRNA isolated from dust mites as template and specific primers, followed by PCR amplification . mRNA was isolated from 100 mg of D. dust mite bodies (Laboratiorios Leti, Madrid, Spain) using the Quick Prep MicroRNA Purification Kit (GE-Healthcare). cDNA was obtained by reverse transcription of mRNA using the First Strand cDNA Synthesis Kit (GE-Healthcare).
[0205] Primers consist of hybridization regions, various cleavage sites for different restriction enzymes (underlined below) and anchor nucleotides. The PCR amplification reaction had the following components in a reaction volume of 50 μl: Amplification buffer x 10, 5 μl; 200 μm dNTPs; 100 μm various oligonucleotide primers; 2.5 units of Taq polymerase (PfxDNA polymerase, Invitrogen); 1 ng DNA Template and make up to 50 μl of sterile...
Embodiment 3
[0212] Example 3: Expression and purification of recombinant Der p 2
[0213] The corresponding plasmids were transformed by the Hanahan method [(21) Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557-580] E. coli BL21(DE3) cells were plated on Petri plates containing LB medium supplemented with 200 μg / ml ampicillin. 50 ml of the same medium were pre-seeded from the cell colonies and incubated overnight at 37°C with stirring (260 rpm). The pre-inoculum was used to inoculate 1 liter of the same medium, starting from an optical density of 0.2 (600 nm). It was incubated at 37°C with stirring until an optical density (600 nm) of 0.6 was reached (approximately 90 minutes), at which point induction was performed with isopropyl-thio-β-galactoside (IPTG) at a final concentration of 0.6 mM . After an induction period of 3 hours, cells were collected by centrifugation.
[0214]Cells were centrifuged at 10000 rpm for 15 minutes a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com