Rapid high-throughput screening model for antiviral drugs
A high-throughput, drug technology, applied in the direction of antiviral agents, pharmaceutical formulations, and microorganism determination/inspection. efficiency, simplify screening procedures, and improve reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
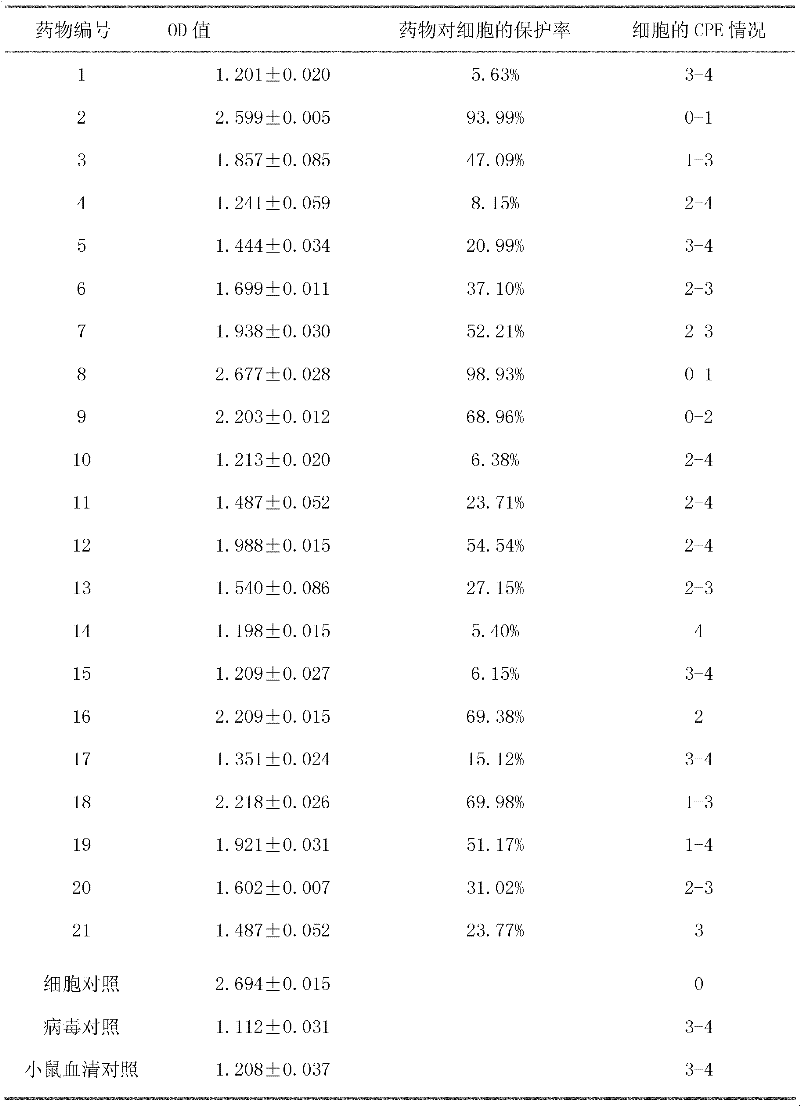
[0025] Determination of Toxic Effects of Chinese Herbal Medicine Extracts on Cultured Cells
[0026] In order to understand and compare the toxic effects of direct drug addition on cells by conventional methods, the toxic effects and safe concentrations of water extracts of 21 Chinese herbal medicines used in the test on Marc-145 cells were measured. The method is to roughly extract 21 kinds of Chinese herbal medicines with water respectively. The water extracts of each Chinese herbal medicine are sterilized and diluted with cell maintenance solution, and added to 96-well culture plates full of cells, with 8 wells per concentration and 100ul per well. 37°C 5% CO 2 Cultivate in the incubator for 72h, observe CPE every day. The results show that various extracts have different degrees of toxic effects on the cells, and the effects increase with the increase of the concentration, and the side effects of different Chinese herbal medicines are different. Add 0.625-1.25% (V / V) or m...
Embodiment 2
[0028] Determination of Safe Concentration of Mouse Serum on Marc145 Cells
[0029] Mouse serum was inactivated at 56°C for 30 minutes, diluted to 80% with maintenance solution, sterilized by filtration through a 0.22 μm microporous membrane, and diluted to different concentrations with maintenance solution. The dilution concentrations of mouse serum were 80%, 40%, 20%, 10%, 5%, 2.5%, 1.25%, 0.625%, 0.312%, from high concentration to low concentration were added to well-grown monolayer cells, 100 μl per well, repeated for each concentration In 8 wells, a cell control group was set, and they were cultured in a 37°C incubator for 72 hours. The CPE was observed with an inverted microscope every day and the results were recorded. The final recorded results were regarded as the final results. At the same time, the OD value measured by MTT method was used to calculate the cell viability. The specific operation is as follows: after 72 hours of treatment, suck out the residual mouse ...
Embodiment 3
[0032] Protection of mouse serum containing Chinese herbal medicine against PRRSV virus infection in Marc-145 cells and screening of antiviral drugs
[0033] 1 test material
[0034] Marc-145 cells (African rhesus monkey kidney cells): donated by the School of Veterinary Medicine, Hunan Agricultural University;
[0035] American porcine reproductive-respiratory syndrome virus (PRRSV): donated by the School of Veterinary Medicine, Hunan Agricultural University;
[0036] Chinese herbal medicine: 21 kinds of Chinese herbal medicine (Guanzhong, Zhonglou, betel nut, star anise lotus, Eucommia, Radix Isatidis, Phellodendron, Aracea, Pinellia, Folium Isatidis, Zihuadiding, Xuanhu, Scutellaria barbata, Cornus officinalis, Forsythia, Cyanorrhizae, oregano, verbena, magma fruit, cnidium, nuxychia, etc.) were purchased from Hunan Yangtianhe Pharmacy, extracted three times by traditional decoction, combined and concentrated, filtered and stored at 4°C;
[0037] 2 test method
[0038] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com