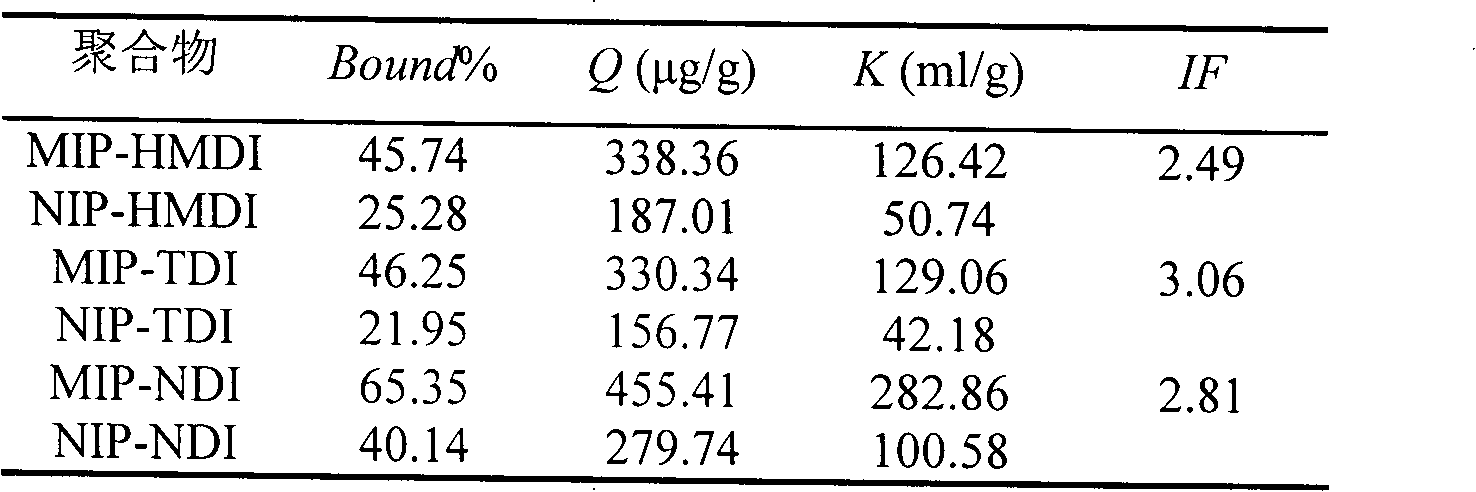Preparation method of cyhalothrin molecularly imprinted polymer and use thereof
A technology of cyhalothrin and molecular imprinting, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of poor imprinting efficiency, large difference in MIP performance, large molecular structure, etc., and achieve good regeneration performance and improve specificity. Adsorption capacity, kinetics combined with fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
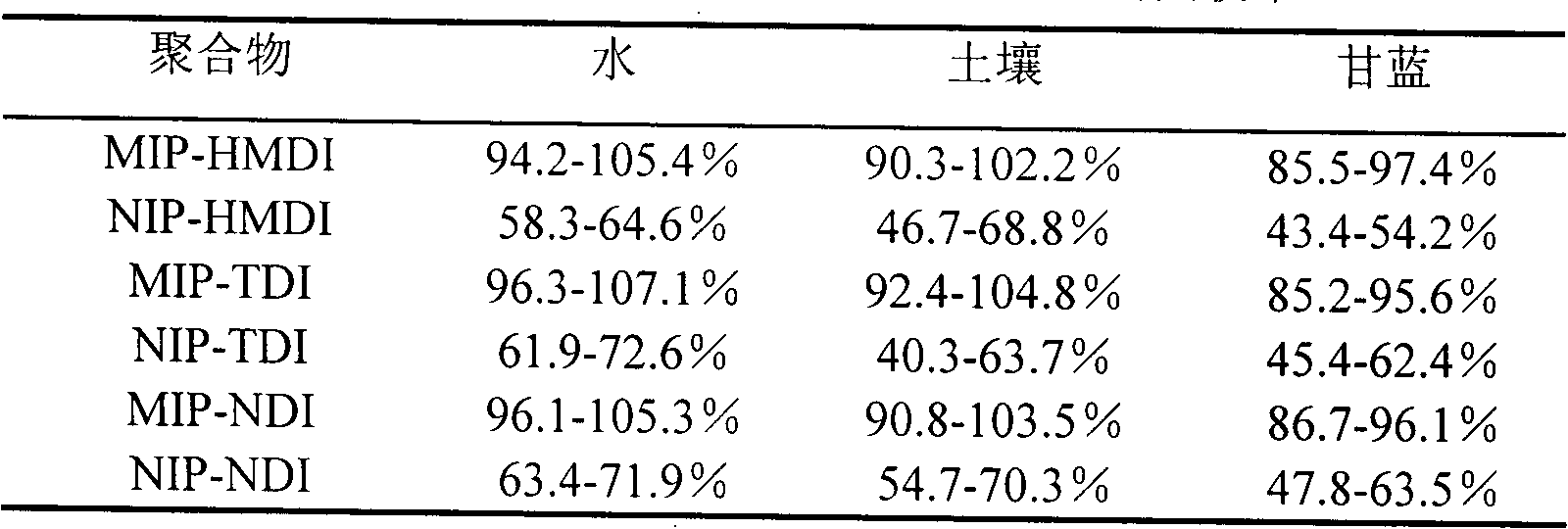
[0022] Weigh 1.5mmol of dry β-cyclodextrin and dissolve it in 20ml of anhydrous dimethyl sulfoxide (DMSO), add 0.5mmol of cyhalothrin (as a template molecule), stir at room temperature (15-35°C) for 1h, and then Slowly add 9mmol of 1,6-hexamethylene diisocyanate (HMDI) dropwise, and react with magnetic stirring at 65°C for 24h; Frequency: 40KHz) to remove template molecules and unreacted β-cyclodextrin, cross-linking agent (1,6-hexamethylene diisocyanate) and DMSO, until no template molecules (cyhalothrin) were detected in the wash supernatant. A GC method can be used to detect whether cyhalothrin as a template molecule is contained in the wash supernatant. After centrifugation, suction filtration and drying, the blocky polymer is ground into fine particles to obtain HMDI-crosslinked cyhalothrin MIP (namely MIP-HMDI), which is stored in a dry container at room temperature.
[0023] Using a non-imprinted polymer (NIP) as a blank control, the synthesis method of the non-imprint...
Embodiment 2
[0038] Weigh 1.5mmol of dry β-cyclodextrin and dissolve it in 20mL of anhydrous DMSO, add 0.5mmol of cyhalothrin (template molecule), stir at room temperature (15-35°C) for 1h, then slowly add 9mmol of toluene-2 , 4-diisocyanate (TDI), magnetic stirring reaction under 65 ℃ for 24h; The flocculent precipitate was then ultrasonically extracted with hot water-ethanol-acetone solvents in order to remove unreacted β-cyclodextrin, cross-linking agent, template molecule and DMSO until no template molecule was detected in the supernatant of the washing solution (using GC detection); after centrifugation, suction filtration, and drying, the massive polymer was ground into fine particles to obtain TDI-crosslinked cyhalothrin MIP (ie MIP-TDI), which was stored in a dry container at room temperature.
[0039] Using NIP as a blank control, the synthesis method is the same as the above-mentioned preparation method of cyhalothrin MIP except that no template molecule is added to obtain NIP-TD...
Embodiment 3
[0053] Weigh 2 mmol of dry β-cyclodextrin and dissolve it in 25 mL of anhydrous DMSO, add 0.5 mmol of cyhalothrin template molecule, stir at room temperature (15-35°C) for 1 hour, then slowly add 10 mmol of naphthalene diisocyanate (NDI) dropwise , and reacted with magnetic stirring at 65°C for 24 hours; after the reaction, the white slurry was taken out and ultrasonically extracted with acetone-hot water-ethanol in order to remove template molecules and unreacted β-cyclodextrin, cross-linking agent (NDI) and DMSO until no template molecules were detected in the supernatant of the washing liquid (detected by GC); after centrifugation, suction filtration, and drying, the blocky polymer was ground into fine particles to obtain NDI-crosslinked cyhalothrin MIP (ie, MIP- NDI), stored in a desiccated container at room temperature.
[0054] Using NIP as a blank control, the synthesis method is the same as the above-mentioned preparation method of cyhalothrin MIP except that no templa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com