ScFv antibody for resisting H5N1 type highly-pathogenic avian influenza and application thereof
A highly pathogenic, H5N1 technology for applications, antibodies, antiviral agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of fully human immune scFv phage antibody library
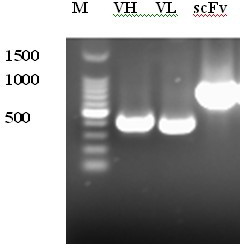
[0030] Collect 40 parts of human whole blood immunized with avian influenza virus vaccine and produce antibodies, separate lymphocytes, use Invitrogen’s Trizol to extract total RNA, and use oligo(dT) 20 As primers, cDNA was generated by reverse transcription. Then use specific primer PCR to amplify the heavy and light chain variable region genes respectively. The scFv gene was synthesized by Overlap PCR ( figure 1 ), was connected with the expression vector pComb3XSS cut with the same restriction enzymes to construct the prokaryotic recombinant expression vector of scFv; electrotransformed competent Escherichia coli XL1-Blue, and constructed a capacity of 6.5×10 8 Fully human immunotype scFv antibody library.
[0031] The connection of the constructed scFv gene with the pComb3xSS carrier refers to: the scFv gene after purification and quantification is subjected to double digestion with SfiI e...
Embodiment 2
[0032] Example 2 Screening, expression and purification of anti-H5N1 highly pathogenic avian influenza virus-specific antibodies
[0033] (1) Coat the solid-phase screening ELISA plate with the hemagglutinin protein HA1 expressed by the purified baculovirus-insect cell expression system, 1 μg per well, wash, add blocking solution, wash, add phage antibody library antibody, wash to remove untreated Bound phage antibody; adding trypsin, eluting specifically bound phage antibody, infection multiplication, assisting phage M13K07 superinfection; repeating the above screening steps, a total of three rounds of "adsorption-elution-amplification" enrichment screening;
[0034](2) Dilute the phage obtained from the last round of screening and multiplication, spread on the culture plate and culture overnight, pick 564 single colonies in the cell culture plate, shake and culture overnight; transfer from each well of the first plate Transfer 5 μL of the bacterial solution to the second pla...
Embodiment 3
[0052] Example 3 Identification of fully human scFv antibodies with binding activity and spectral neutralization activity
[0053] The six H5N1 virus strains A / CK / Hongkong / 3-69 (H5N1), A / DK / Beijing / wy12107 (H5N1), A / Goose / Jilin / 514-1 (H5N1), A / Jiangsu / 07-4 (H5N1), A / Goose / ZD / 8-9106 (H5N1), A / Jiangsu / 08-6 (H5N1), A / Jiangsu / 1 / 2007(H5N1) (GenBank: EU434686.1) Provided by the Jiangsu Provincial Center for Disease Control and Prevention. The RNA of 6 virus strains was extracted, the full length of HA was amplified by RT-PC for sequencing, and the evolutionary tree was constructed using MEGA5.0 software, compared with the H5N1 virus strains provided by WHO, and classified into clade.
[0054] The HA proteins of six H5N1 virus strains were sequenced, analyzed using MEGA5.0 software, and a phylogenetic tree was constructed. The results show that A / CK / Hongkong / 3-69 (H5N1) belongs to clade 9, A / Goose / Jilin / 514-1 (H5N1) belongs to clade 2.2, A / Jiangsu / 07-4 (H5N1), A / Goose / ZD / 8-9106...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com