Process for selectively extracting copper and cobalt from heterogenite
A selective, water-cobalt ore technology, applied in the direction of improving process efficiency, can solve problems such as long processing process flow, and achieve the effects of environmental friendliness, strong selectivity, and fast leaching speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
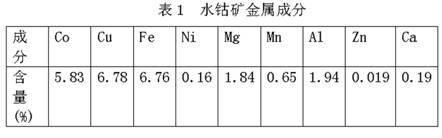
[0014] Example 1: In this example, the metal composition of the cobalt ore used in the water is shown in Table 1.
[0015]
[0016] (1) Acid leaching: Weigh 3000g of water cobalt ore powder with a particle size of less than 60 mesh, add 180g of sodium sulfite and mix well; put it into a self-made leaching tank, add 12000mL of 0.75mol / L sulfuric acid, leaching temperature 90℃, start stirring reaction 1.5 Hours; the concentration of copper, cobalt, and iron in the leaching solution were 16.74 g / L, 14.13 g / L, and 0.08 g / L, respectively, and the leaching rates of copper, cobalt, and iron were 98.76%, 96.95%, and 0.47%, respectively. The iron content in the leaching solution is less than 0.1g / L, which meets the conditions of swirling electrowinning of copper and cobalt;
[0017] (2) The leaching liquid is separated by filtration, the leaching residue is washed with water, and the washing liquid is returned to the acid leaching; the washed leaching residue is stored in a centralized ma...
Embodiment 2
[0021] Embodiment 2: In this embodiment, the metal composition of cobalt ore used in the water is the same as in Table 1.
[0022] (1) Acid leaching: Weigh 3000g of water cobalt ore powder with a particle size of less than 60 mesh, add 240g of sodium sulfite and mix well, put it into a self-made leaching tank, add 12000mL of 1.25mol / L sulfuric acid, and start the stirring reaction at the leaching temperature of 60℃ 2.5 hours. The concentrations of copper, cobalt, and iron in the leaching solution were 16.58g / L, 14.29g / L, and 0.09g / L, respectively. The leaching rates of copper, cobalt, and iron were 98.04%, 97.82%, and 0.53%, respectively. The iron content in the leaching solution is less than 0.1g / L, which meets the conditions of swirling electrowinning of copper and cobalt;
[0023] (2) The leaching liquid is separated by filtration, the leaching residue is washed with water, and the washing liquid is returned to the acid leaching; the washed leaching residue is stored in a cent...
Embodiment 3
[0027] Embodiment 3: In this embodiment, the metal composition of the cobalt ore used in the water is the same as in Table 1.
[0028] (1) Acid leaching: Weigh 3000g of water cobalt ore powder with a particle size of less than 60 mesh, add 300g of sodium sulfite and mix well, put it into a self-made leaching tank, add 18000mL of 1.75mol / L sulfuric acid, and start the stirring reaction at the leaching temperature of 90℃ 1.5 hours. The concentrations of copper, cobalt, and iron in the leaching solution were 11.07g / L, 9.55g / L, and 0.07g / L, respectively. The leaching rates of cobalt, copper, and iron were 98.19%, 98.05%, and 0.62%, respectively. The iron content in the leaching solution is less than 0.1g / L, which meets the conditions of swirling electrowinning of copper and cobalt;
[0029] (2) The leaching liquid is separated by filtration, the leaching residue is washed with water, and the washing liquid is returned to the acid leaching; the washed leaching residue is stored in a ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com