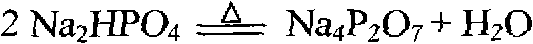Method for removing chromium impurities from nickel leach liquor
A technology for leaching liquid and impurities, which is applied in the field of removing chromium impurities from nickel leaching liquid, and can solve the problems of poor nickel taste, and achieve the effects of less nickel loss rate, pollution elimination, and high economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
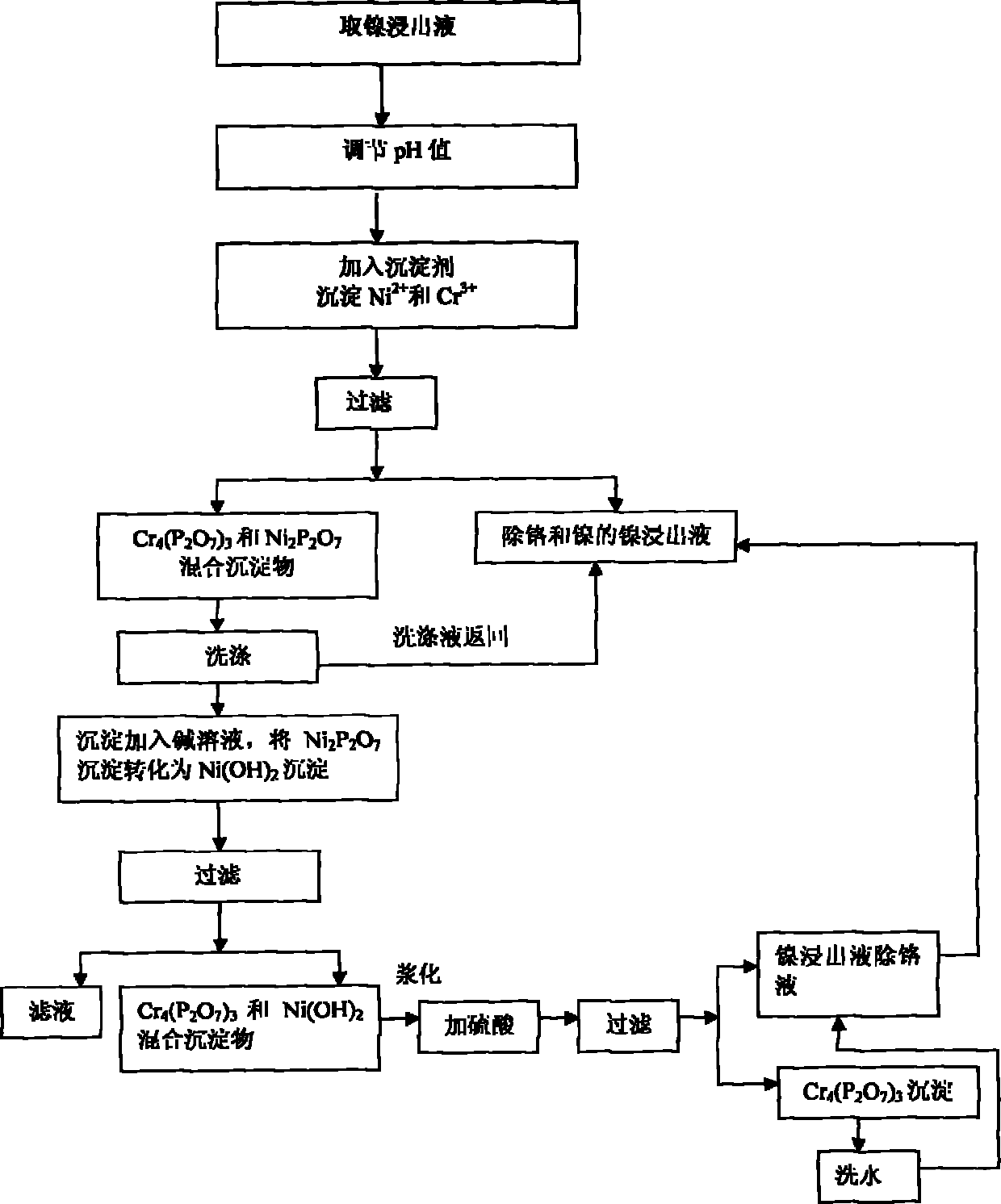
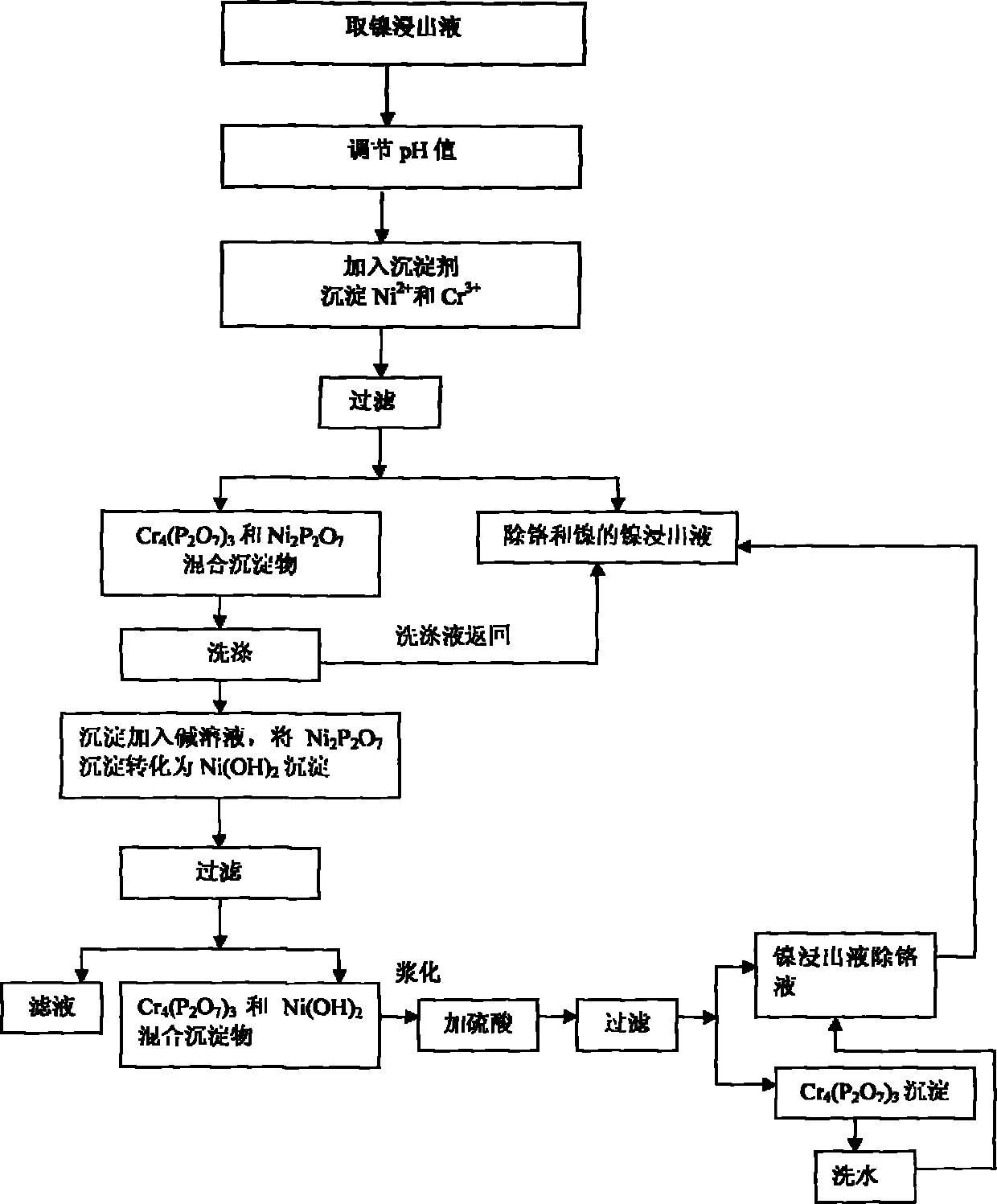
[0031] Take 500mL nickel concentration is 36.70g / L, chromium concentration is 8.78g / L, initial pH value is 1.0 nickel leaching solution I; Add the NaOH solution that 46mL concentration is 4.73mol / L, adjust the initial pH value of nickel leaching solution I to be 2.5 .
[0032] At a temperature of 70°C, add 18.83g of sodium pyrophosphate (excess coefficient 1.0), stir and react for 1 hour, filter to obtain chromium pyrophosphate (Cr 4 (P 2 o 7 ) 3 ) and nickel pyrophosphate (Ni 2 P 2 o 7 ) mixed precipitate I and 365mL nickel leaching solution II with a nickel concentration of 23.35g / L and a chromium concentration of 1.71g / L for the removal of chromium and nickel. In addition, chromium pyrophosphate (Cr 4 (P 2 o 7 ) 3 ) and nickel pyrophosphate (Ni 2 P 2 o 7 ) Mixing the precipitate I to obtain 2850mL of washing solution III with a nickel concentration of 3.64g / L and a chromium concentration of 0.074g / L. Chromium pyrophosphate (Cr 4 (P 2 o 7 ) 3 ) and nickel py...
Embodiment 2 Embodiment 10
[0036] The method is the same as in Example 1, and 500 ml of nickel leach solution I with a nickel concentration of 36.70 g / L, a chromium concentration of 8.78 g / L and an initial pH value of 1.0 is taken. See Table 1 for the differences in parameters in the technical solutions of Embodiment 2 to Embodiment 10.
[0037] Table 1. Differences in parameters in the technical solutions of Embodiment 2 to Embodiment 10
[0038]
[0039]
[0040] in conclusion
[0041] After adjusting the pH value of nickel leaching solution and adding precipitant to precipitate Cr 3+ In the process, chromium can be effectively removed by reasonably controlling the pH value, the amount of precipitant used, and the reaction temperature and time, and the removal effect of chromium is obvious. When the excess coefficient of sodium pyrophosphate is low (1.0), the removal rate of chromium reaches more than 83%, and when the excess coefficient of sodium pyrophosphate increases to 1.6, the removal ra...
Embodiment 11
[0043] The impurity-removing dry slag I after the aforementioned washing is dried and ground into a uniform fine powder. In the powder, the nickel content is 12.69%, the chromium content is 7.57%, and the phosphorus content is 11.2%.
[0044] Take 30g of fine powder and add 300mL of tap water to make a slurry, add it to 10mL of NaOH solution with a concentration of 5mol / L, and adjust the pH value to 9.0. Stir and react at 80°C for 2 hours, filter to obtain 340ml of phosphorus-containing solution IV and chromium pyrophosphate (Cr 4 (P 2 o 7 ) 3 ) and nickel hydroxide (Ni(OH) 2 ) Mixed Precipitate II. Add the mixed precipitate II to 300ml of bottom water, and then add 15mL of H with a concentration of 6mol / L. 2 SO 4 solution, adjust the pH to 2.0. Heat up to 80° C., stir and react for 1 hour, and filter to obtain 440 ml of chromium-removing solution V and chromium pyrophosphate slag III of nickel leaching solution with a nickel concentration of 5.35 g / L and a chromium con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com