Biphenyl compound serving as antitumor medicament and preparation method thereof
A technology of anti-tumor drugs and compounds, which is applied in the field of biomedicine, can solve the problems of unanticipated tumor chemotherapy, toxic and side effects of chemotherapy drugs, etc., and achieve the effect of cheap and easy-to-obtain reagents, simple operation of the reaction process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
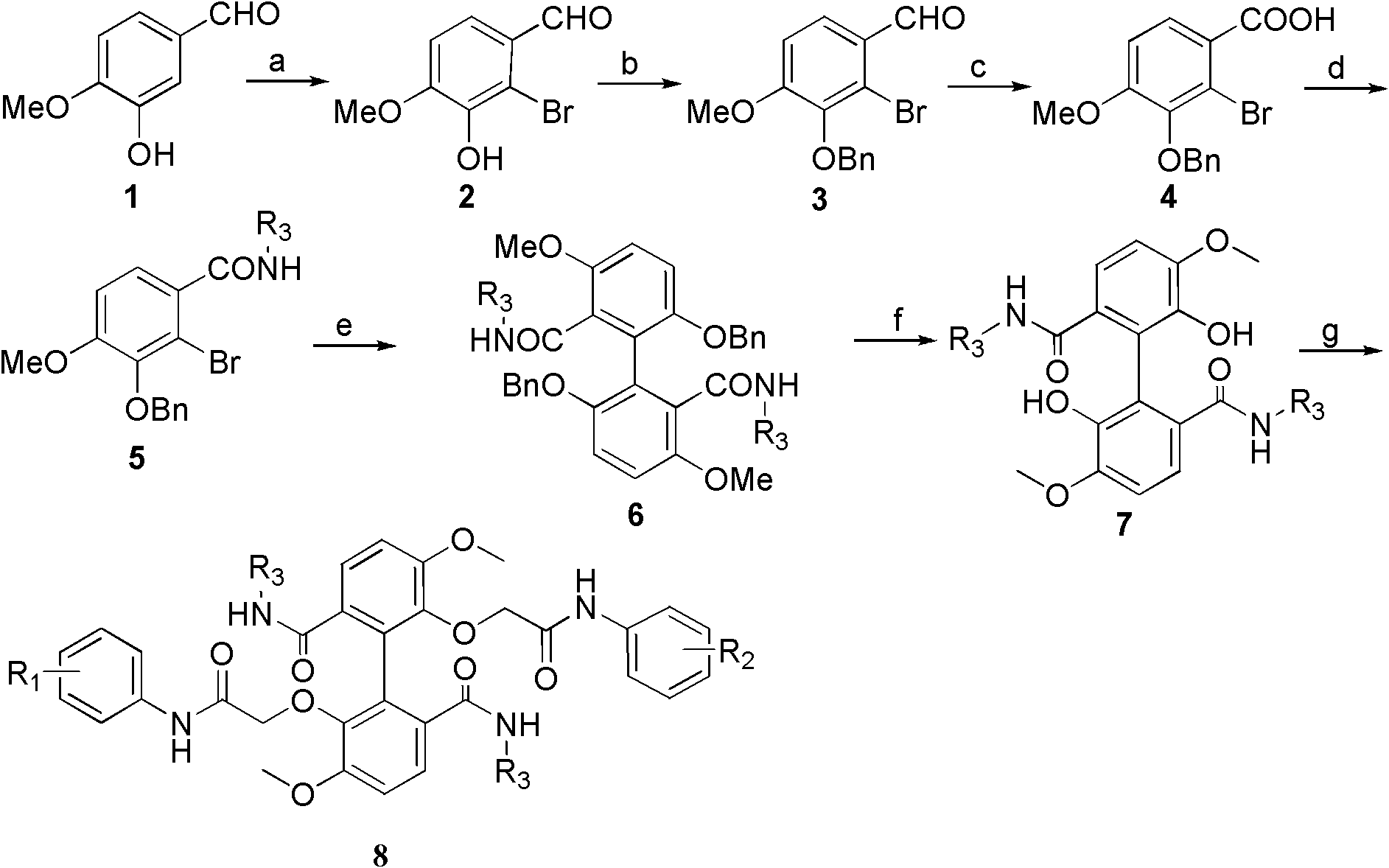
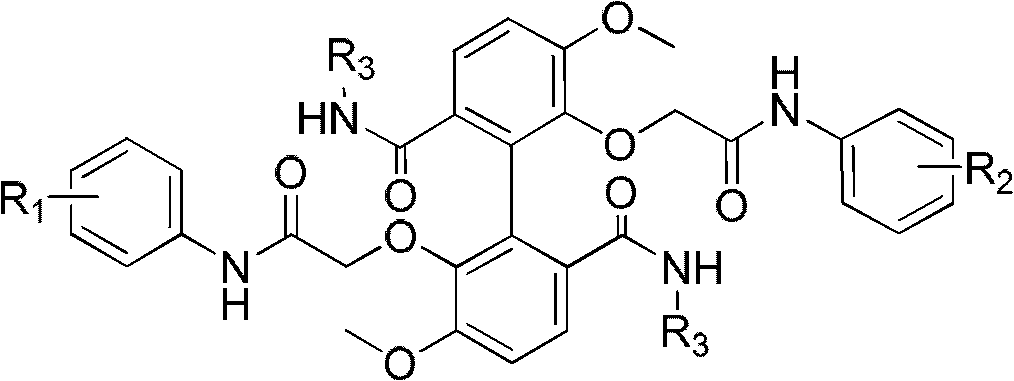
[0047] Example 1 R in the structural formula 1 , R 2 Both are 3-chloro-4-fluoro substituted, R 3 Be the compound of diethyl, prepare by the following steps:
[0048] Preparation of 2-chloro-N-(3-chloro-4-fluorophenyl)acetamide
[0049] 1) Isovanillinization (1) Preparation of compound 2-bromoisovanillin (2) by bromination reaction
[0050] Put 20.0g (0.132mol) of isovanillin (1), 21.59g (0.263mol) of sodium acetate and 0.68g (0.012mol) of iron powder into a 500mL three-necked flask, add 120ml of glacial acetic acid, and stir at room temperature for 30min;
[0051] After the stirring is completed, add dropwise the solution prepared by mixing 7.0mL (0.14mol) liquid bromine and 30mL glacial acetic acid in advance under the condition that the temperature is controlled at 23-25°C. ;
[0052] Then add 250 mL of ice water, stir for 1 h; filter, evaporate the solid to dryness, and recrystallize from ethanol to obtain 24.59 g of an off-white solid product of 2-bromoisovanillin (2)...
Embodiment 2
[0081] Example 2 R in the structural formula 1 , R 2 Both are substituted with 4-trifluoromethyl, R 3 Be the compound of diethyl, prepare by the following steps:
[0082] Steps 1) to 6) are the same as in Example 1, that is, from compound isovanillinization (1) to compound 5,5'-dimethoxy-6,6'-dihydroxybiphenyl-2,2'- The preparation steps of diformyl diethylamide (7) are the same; afterwards, in two different phenolic hydroxyl etherification processes, two phenolic hydroxyl chloroacetyl 4-trifluoromethylaniline reactions are specifically:
[0083] Dissolve 3.90g (10mmol) of compound 5,5'-dimethoxy-6,6'-dihydroxybiphenyl-2,2'-diformyldiethylamide in (7) 200mL of anhydrous acetone, add 8.30 g (60 mmol) of anhydrous potassium carbonate, stirred at room temperature for 30 minutes, then added 5.21 g (22 mmol) of 2-chloro-N-(4-trifluoromethylbenzene) acetamide, and heated to reflux at 56°C for 8 hours;
[0084] After the reaction is complete, evaporate the solvent under reduced p...
Embodiment 3
[0088] Example 3 R in the structural formula 1 , R 2 Both are 3-chloro-4-fluoro substituted, R 3 Compounds substituted with isopropyl groups are prepared by the following steps:
[0089] Steps 1) to 3) are the same as in Example 1, after which this example prepares compound 4-methoxy-3 through a two-step reaction with 4-methoxy-3-benzyloxy-2-bromobenzoic acid (4) -benzyloxy-2-bromobenzoylisopropylamide (5), specifically:
[0090] 5.04g (0.015mol) of 4-methoxy-3-benzyloxy-2-bromobenzoic acid (4) was dissolved in 50mL of dichloromethane, and 4.37mL (0.060mol) of thionyl chloride and Catalytic amount of N,N-dimethylformamide (DMF), stirred at room temperature for 3h; after the reaction was complete, the solvent was evaporated under reduced pressure, and the compound containing The residue of benzoyl chloride was dissolved in 25 mL of anhydrous dichloromethane;
[0091] Under ice-bath conditions, the obtained above solution was added dropwise to 2.58mL (0.030mmol) of isopropy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com