Tert-butyl carbamate derivative and preparation method and application thereof
A technology of isobutyl chloroformate and serine, applied in the fields of organic chemistry and medicinal chemistry, can solve problems such as large loss, great difficulty, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
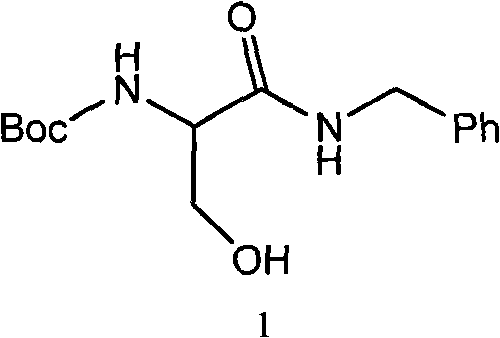
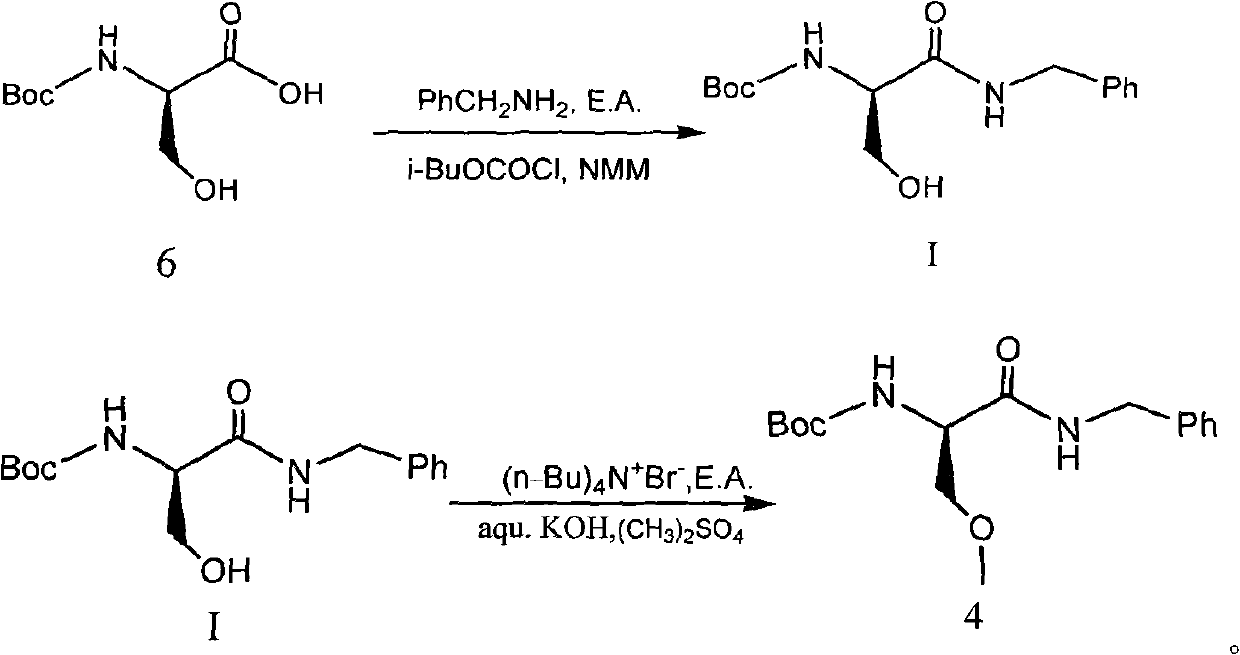
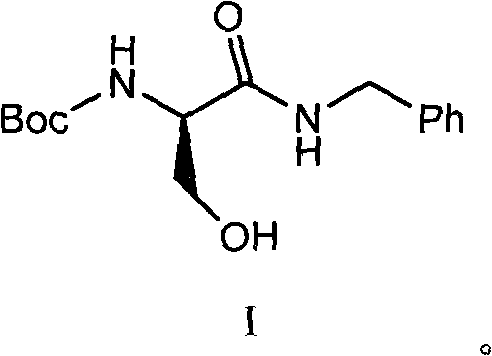
[0065] 29.48g benzylamine was dissolved in 50g anhydrous ethyl acetate to prepare a solution for use. In a 1000ml four-necked round bottom flask, 51.3g of compound 6 (N-BOC-D-serine) was dissolved in 400ml of anhydrous ethyl acetate, the temperature was lowered to -10℃, and 27.83g of N-methylmorpholine and 37.59g isobutyl chloroformate. After the addition is complete, continue the reaction at -15~-10°C for 2 hours. Then add dropwise the ethyl acetate solution of benzylamine that has been prepared. After the dropwise addition is completed, the temperature is raised to 10-15°C and reacted for 2 hours. After the reaction is completed, add water, extract the phases, wash the organic phase with dilute hydrochloric acid and brine, evaporate the solvent under reduced pressure, crystallize with hexane / ethyl acetate = 8 / 1, and dry to obtain 66.3 g of the product with a yield of 90.2% .
[0066] The structure data is as follows: 1H NMR (400MHz, DMSO- ) ppm: 1.38 (s, 9H), 3.58 (m, 2H), ...
Embodiment 2
[0068] 34.84g benzylamine was dissolved in 60g anhydrous ethyl acetate to prepare a solution for use. In a 1000ml four-necked round bottom flask, 51.3g of compound 6 (N-BOC-D-serine) was dissolved in 400ml of anhydrous ethyl acetate, the temperature was lowered to -20℃, and 32.99g of N-methylmorpholine and 44.42g isobutyl chloroformate. After the addition is complete, continue the reaction at -15~-10°C for 2 hours. Then add dropwise the ethyl acetate solution of benzylamine that has been prepared. After the dropwise addition is completed, the temperature is raised to 15-20°C and reacted for 2 hours. After the reaction is complete, add water, extract the phases, wash the organic phase with dilute hydrochloric acid and brine, evaporate the solvent under reduced pressure, crystallize with hexane / ethyl acetate = 8 / 1, and dry to obtain 66.9 g of product with a yield of 91.0% .
Embodiment 3
[0070] 40.20 g benzylamine was dissolved in 70 g anhydrous ethyl acetate to prepare a solution for use. In a 1000ml four-necked round bottom flask, 51.3g of compound 6 (N-BOC-D-serine) was dissolved in 400ml of anhydrous ethyl acetate, the temperature was reduced to 0℃, and 38.06g of N-methylmorpholine and 51.25 were added dropwise successively. g Isobutyl chloroformate. After the dropwise addition is completed, continue the reaction at 0-5°C for 2 hours. Then add dropwise the ethyl acetate solution of benzylamine that has been prepared. After the dropwise addition is completed, the temperature is raised to 10-15°C and reacted for 2 hours. After the reaction is complete, add water, extract the phases, wash the organic phase with dilute hydrochloric acid and brine, evaporate the solvent under reduced pressure, crystallize with hexane / ethyl acetate = 8 / 1, and dry to obtain 68.4 g of product with a yield of 93.1% .
[0071] Compound 4, the synthesis of (R)-2-(amino-Boc)-N-benzyl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com