Immunochromatographic test strip for full quantitative detection of C-reactive protein and preparation method thereof
An immunochromatographic test strip and quantitative detection technology, applied in the field of medical testing, can solve problems such as inability to diagnose infectious diseases or observation of curative effects, inability to be used as a risk indicator for cardiovascular and cerebrovascular diseases, inability to accurately measure the content of C-reactive protein, etc. , to achieve good linear range, accurate identification of infection, and good labeling stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In the embodiment of the present invention, the CRP antibody used is a monoclonal antibody prepared by conventional monoclonal antibody technology, and the principle of detecting CRP antigen by the double-antibody sandwich method is used to detect the specimen.
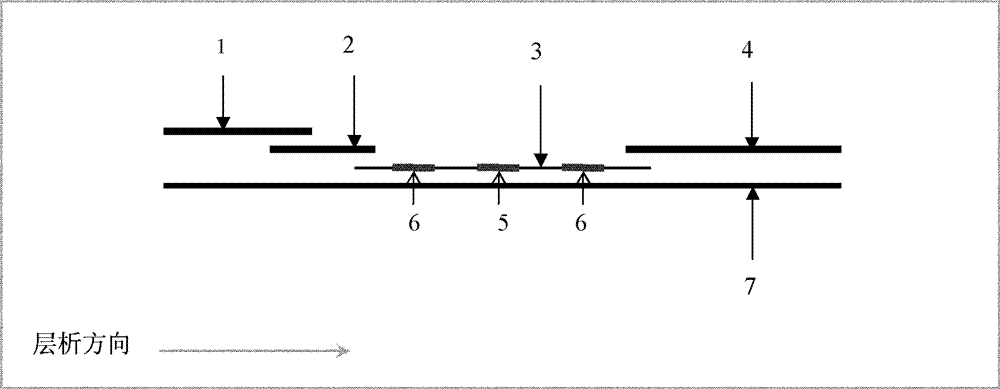
[0037] Such as figure 1 As shown, in this embodiment, the CRP immunochromatographic test strip is quantitatively detected throughout the whole process, including the far end and the proximal end. The sample pad 1 is located at the proximal end of the test strip, and it contains a hydrophilic porous membrane. The sample Pad 1 is a sample loading area, which is used to absorb the CRP detection sample to be detected. Between the sample pad 1 and the distal end, a marker pad 2 made of glass cellulose membrane, a coating membrane 3 made of nitrocellulose membrane and absorbent paper 4 are overlapped in sequence. The sample pad 1 , marker pad 2 , coating film 3 and absorbent paper 4 are all set on the bottom plate 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com