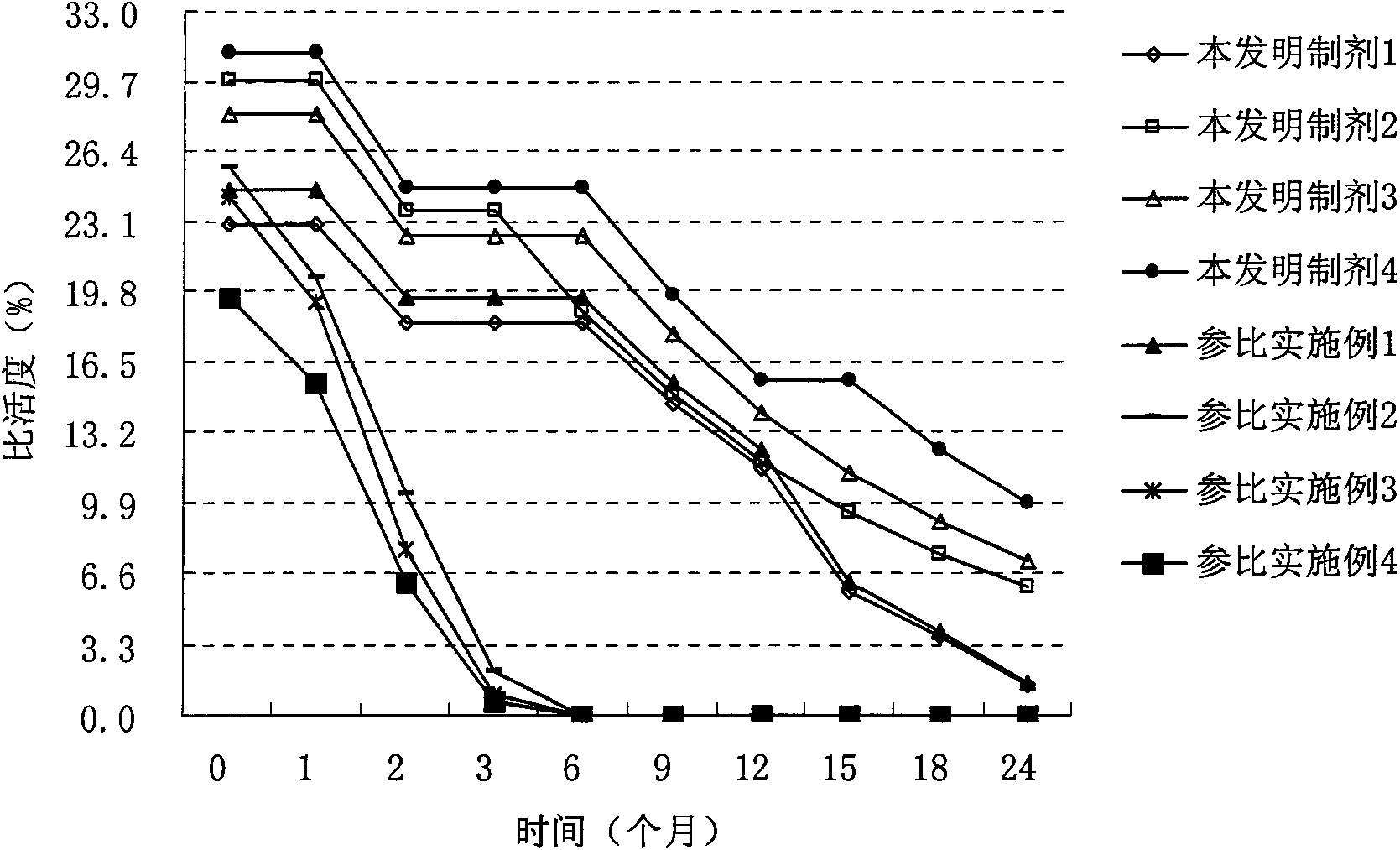
Preparation of recombinant adenovirus
A recombinant adenovirus and preparation technology, which is applied in the field of preparations, can solve problems such as the inability to use adenovirus preparations and the inability to maintain the activity of recombinant adenoviruses, and achieve good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
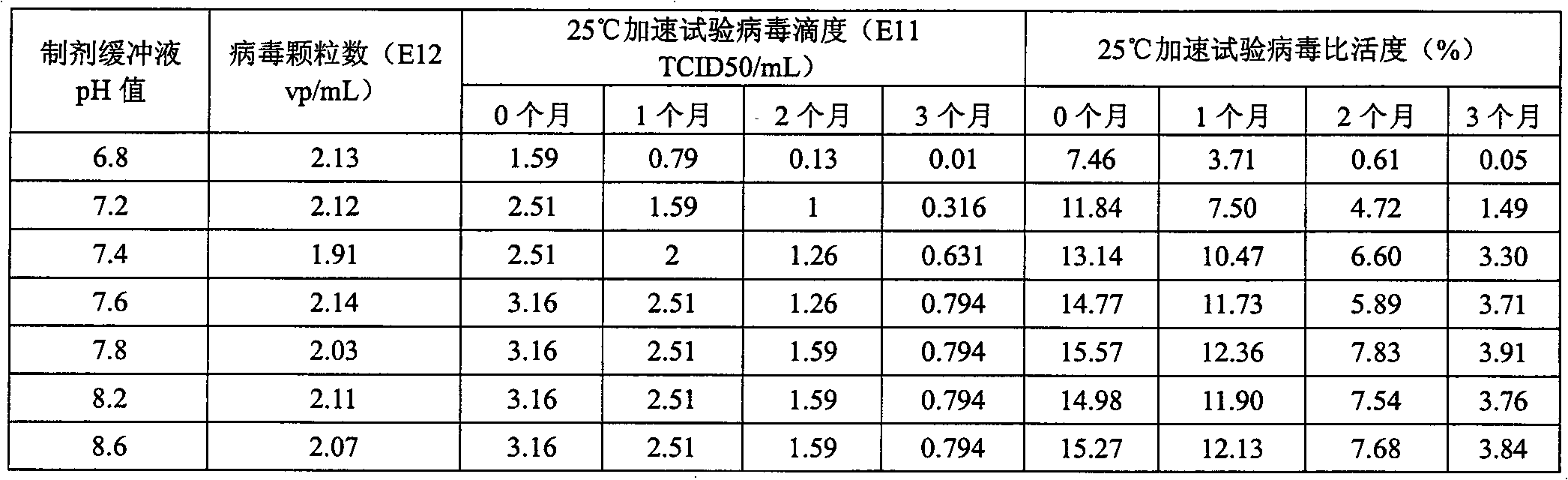
[0045] Example 1 Effect of pH value on the stability of recombinant adenovirus in preparation
[0046] In order to study the effect of pH value on the stability of recombinant adenovirus in the preparation, at 25°C, we studied the range of pH 6.8-8.6, and the pH values were 6.8, 7.2, 7.4, 7.6, 7.8, 8.2, and 8.6. , the effect of pH on the stability of recombinant adenoviruses in preparations.
[0047] By detecting the virus titer (TCID50 / mL) and specific activity of the recombinant adenovirus in the preparation, the effect of pH value on the stability of the recombinant adenovirus in the preparation was studied, and the results are shown in Table 1.
[0048] The influence of table 1pH value on the stability of recombinant adenovirus in the preparation
[0049]
[0050] Note: The composition of the preparation is 10mM Tris+10w / v% propylene glycol, and the pH is adjusted with HCl.
[0051] It can be seen from Table 1 that when the pH is lower than 7.4, the recombinant ad...
Embodiment 2
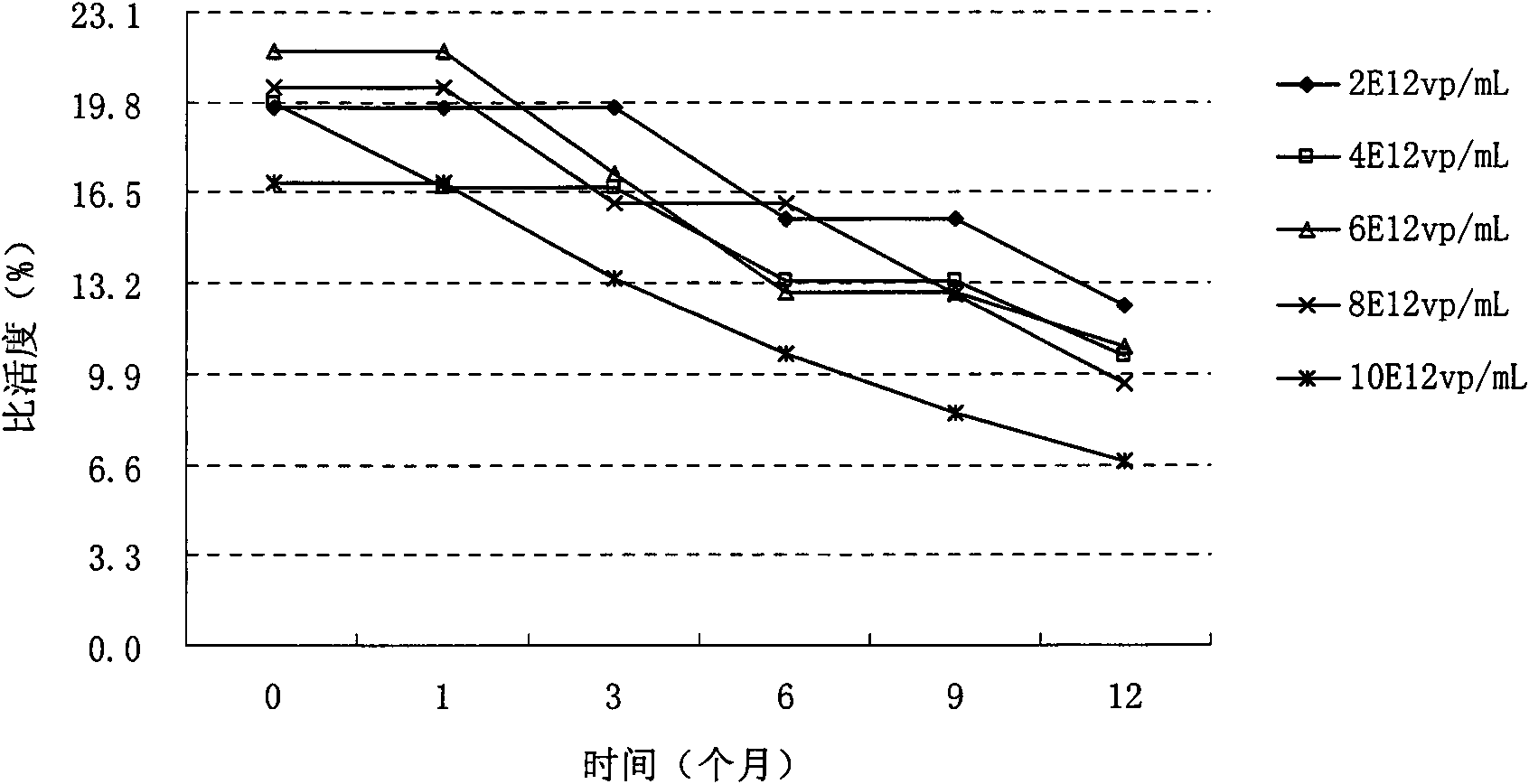
[0052] Example 2 Effect of protective agent on the stability of recombinant adenovirus in preparation
[0053] The effects of protective agents (propylene glycol, glycerol, polyethylene glycol 400, dimethyl sulfoxide, sorbitol, mannitol, sucrose, trehalose) on the stability of recombinant adenovirus in the preparation were studied at 25°C . Wherein, the control preparation is ARCA preparation.
[0054] By detecting the virus titer (TCID50 / mL) and specific activity of the recombinant adenovirus in the preparation, the influence of the protective agent on the stability of the recombinant adenovirus preparation in the preparation was studied. The composition and research results of the preparation are shown in Table 2.
[0055] The impact of table 2 protective agent on the stability of recombinant adenovirus in the preparation
[0056]
[0057]
[0058] Note: The Tris concentration in the Tris-HCl buffer solution is 10mM, and the pH is adjusted to 7.8 with HCl during pr...
Embodiment 3
[0060] Example 3 Effect of Amino Acids on the Stability of Recombinant Adenovirus in Preparation
[0061] Under the condition of 25°C, the present invention studies the influence of amino acids (glycine, histidine, lysine, arginine, methionine, proline) on the stability of the recombinant adenovirus in the preparation.
[0062] By detecting the virus titer (TCID50 / mL) and specific activity of the recombinant adenovirus in the preparation, the influence of amino acids on the stability of the recombinant adenovirus preparation in the preparation was studied. The composition and research results of the preparation are shown in Table 3.
[0063] The impact of table 3 amino acids on the stability of recombinant adenovirus in the preparation
[0064]
[0065] Note: Tris concentration in Tris-HCl buffer is 10mM, adjust pH to 7.8 with HCl during preparation; propylene glycol concentration is 10w / v%.
[0066] It can be seen from Table 3 that compared with the preparation without ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com