Method for selectively depositing thin film on substrate by utilizing atomic layer deposition
An atomic layer deposition, selective technology, used in coatings, metal material coating processes, gaseous chemical plating, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
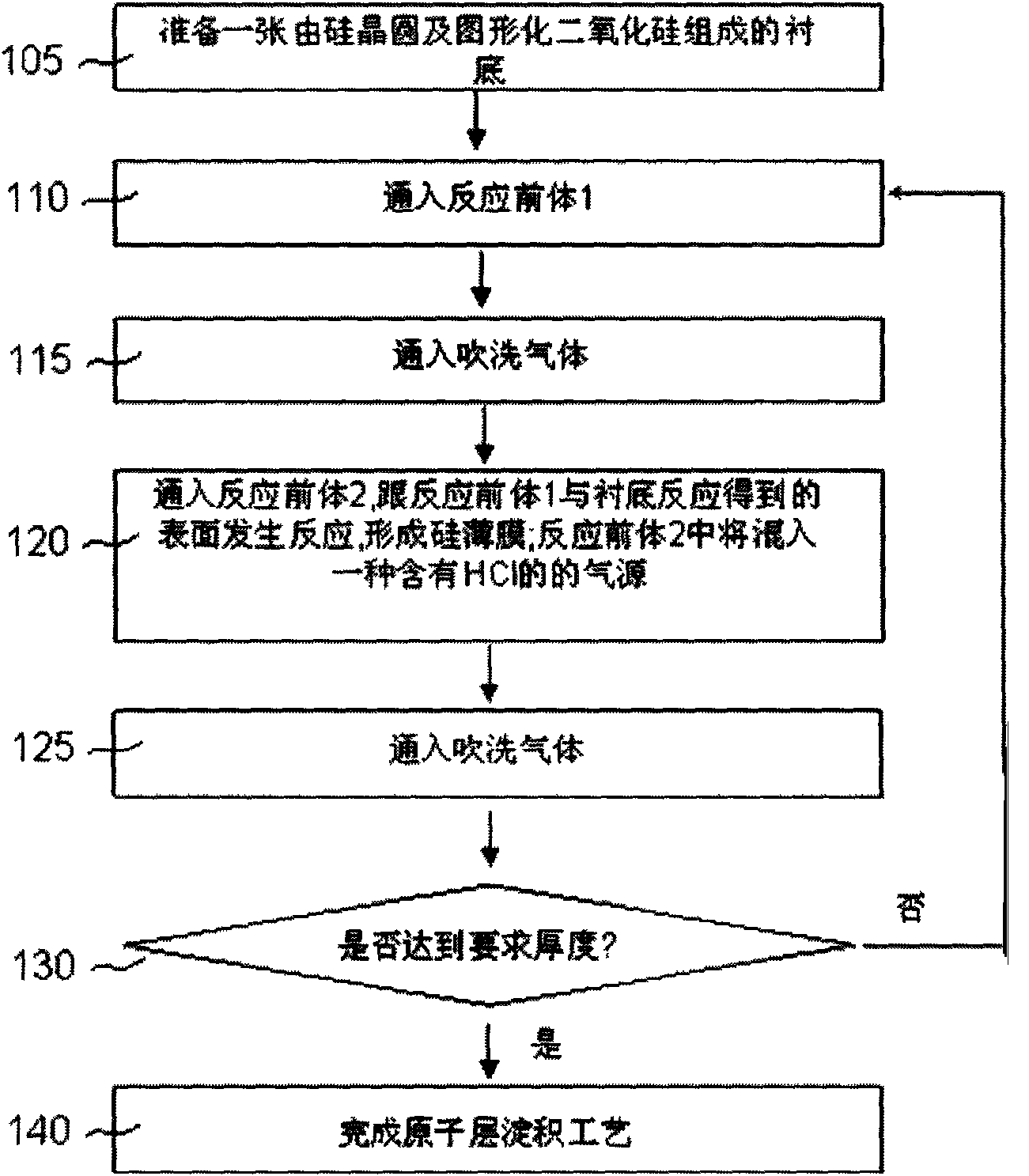
[0032] figure 1 It is a process flow diagram of selective deposition of silicon and germanium by atomic layer deposition. The oxides mentioned in the figure can be replaced by nitrides in some applications. First, the substrate is placed in the ALD reaction chamber. At 110, a silicon-containing reactive precursor, such as SiH2Cl2 or SiCl4, is introduced at the substrate. SiH2Cl2 or SiCl4 will be adsorbed on the substrate surface. Then, at 115, a purge gas is introduced into the reaction chamber. Nitrogen, argon or other inert gases can be used as purge gas. At 120, a second reactive precursor is passed into the reaction chamber. This reactive precursor can be SiH4 or GeH4. The second reaction precursor reacts with the Si-Cl group or other groups formed on the surface of the first reaction precursor to form HCl and the silicon or silicon germanium film we need. exist figure 1 In , selective deposition is achieved by mixing HCl gas in the second reactive precursor. HCl ...
Embodiment 2
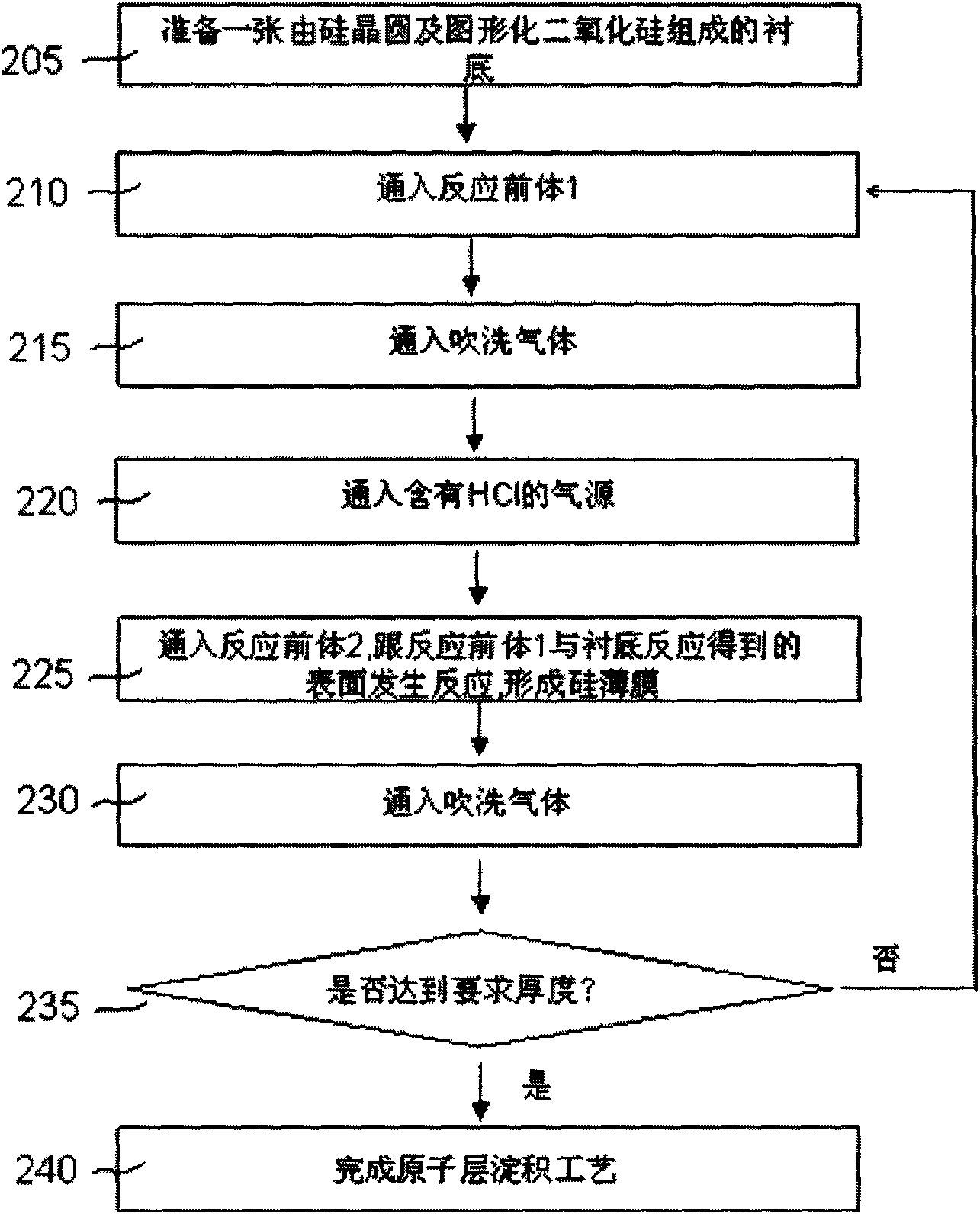
[0035] figure 2 It is the process flow diagram of the second selective deposition of silicon and germanium by atomic layer deposition.
[0036] First, with figure 1 Similar to the above, the process flow starts from 205, that is, a substrate including a semiconductor wafer and a patterned oxide. In some applications, the oxides can be replaced by nitrides. Treated substrates with figure 1 In the same way, it is placed in the atomic layer reaction chamber. At 210, silicon-containing reactive precursors, such as SiH2Cl2 and SiCl4, are passed into the reaction chamber to reach the substrate surface. SiH2Cl2 and SiCl4 will be adsorbed on the substrate surface. Then, at 215, a purge gas is introduced into the reaction chamber. Nitrogen, argon or other inert gases can be used as purge gas. follow Figure 1 The difference in is that figure 2 In , the HCl gas is not introduced into the reaction chamber together with the reaction precursor 2, but the introduction of HCl is r...
Embodiment 3
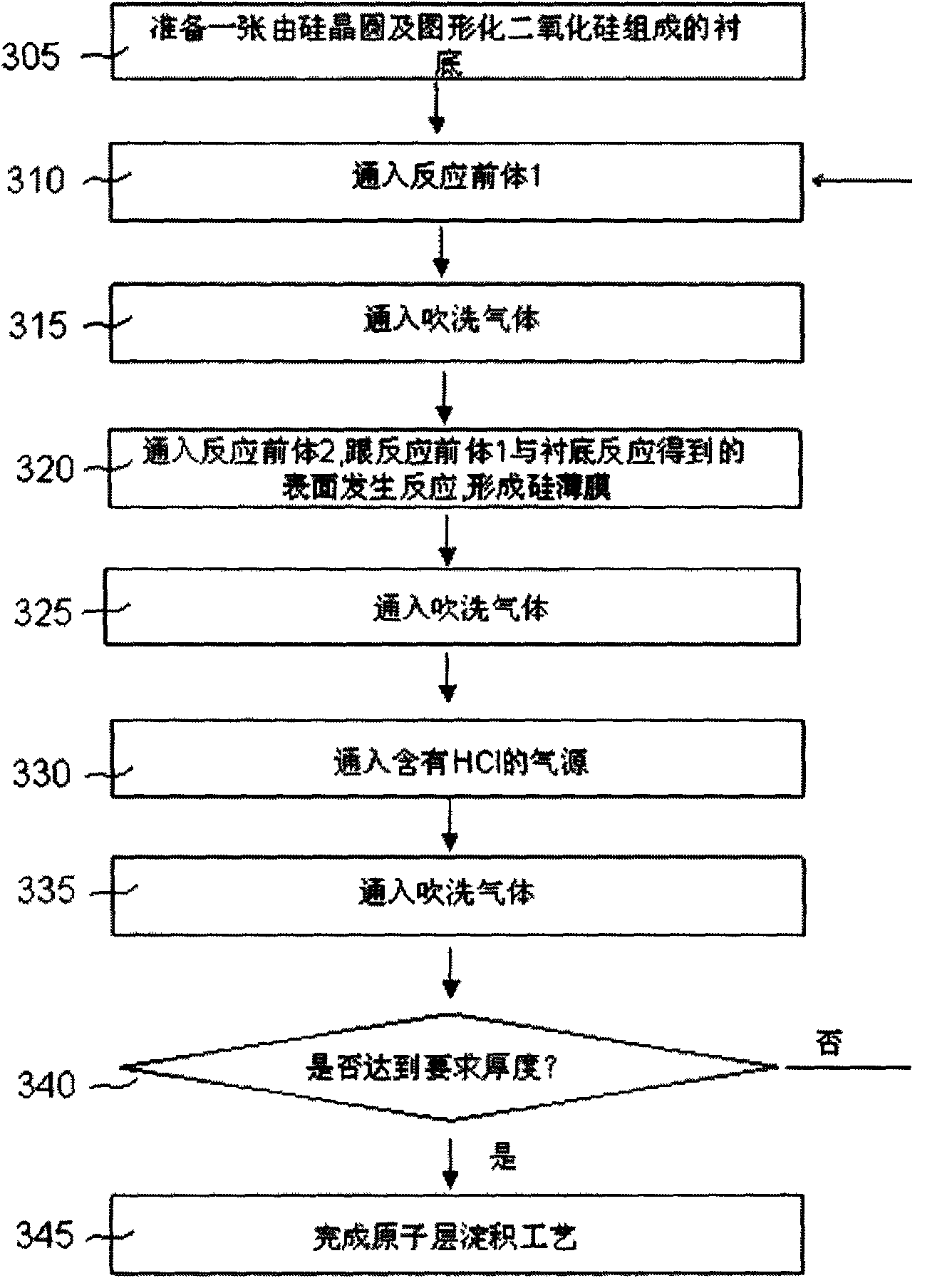
[0038] image 3 It is the process flow diagram of the third selective deposition of silicon and germanium by atomic layer deposition. like figure 1 In , the process flow starts from 305, a substrate including a semiconductor wafer and patterned oxide. In some applications, the oxide inside can be replaced by a nitride. Treated substrates with figure 1 It is placed in the atomic layer reaction chamber as in the process. At 310, silicon-containing reactive precursors, such as SiH2Cl2 and SiCl4, are passed into the reaction chamber to reach the substrate surface. SiH2Cl2 and SiCl4 will be adsorbed on the substrate surface. Then, at 315, a purge gas is introduced into the reaction chamber. Nitrogen, argon or other inert gases can be used as purge gas. At 320, a second reactive precursor is passed into the reaction chamber. This reaction precursor can be SiH4 or SiH2Cl2. The second reaction precursor reacts with the Si-Cl group or other groups formed on the surface of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com