Nitrogen-doped graphite carbon serving as anode material of lithium ion battery, and preparation method and application thereof
A graphite carbon and nitrogen doping technology, applied in battery electrodes, circuits, electrical components, etc., to achieve the effects of low cost, easy large-scale production, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the nitrogen-doped graphite carbon negative electrode material of the present invention is to use the graphite carbon material as a raw material, place it in a nitrogen-containing small molecule material or a nitrogen-containing small molecule material atmosphere and calcinate for nitriding treatment to obtain lithium ions Nitrogen-doped graphitic carbon as battery anode material.
[0027] The graphite carbon raw material of the present invention is graphite materials such as graphene oxide, reduced graphene, natural graphite, artificial graphite, expanded graphite, or mesocarbon microspheres.
[0028] The nitrogen-containing small molecule material of the present invention is one or more of reactive substances such as pure ammonia, organic amine, organic amine salt, nitrogen, etc., and its volume percentage is 1-99%.
[0029] The nitriding temperature of the present invention is 300-1100° C., and the nitriding time is 0.1-72 hours.
[0030] T...
Embodiment 1
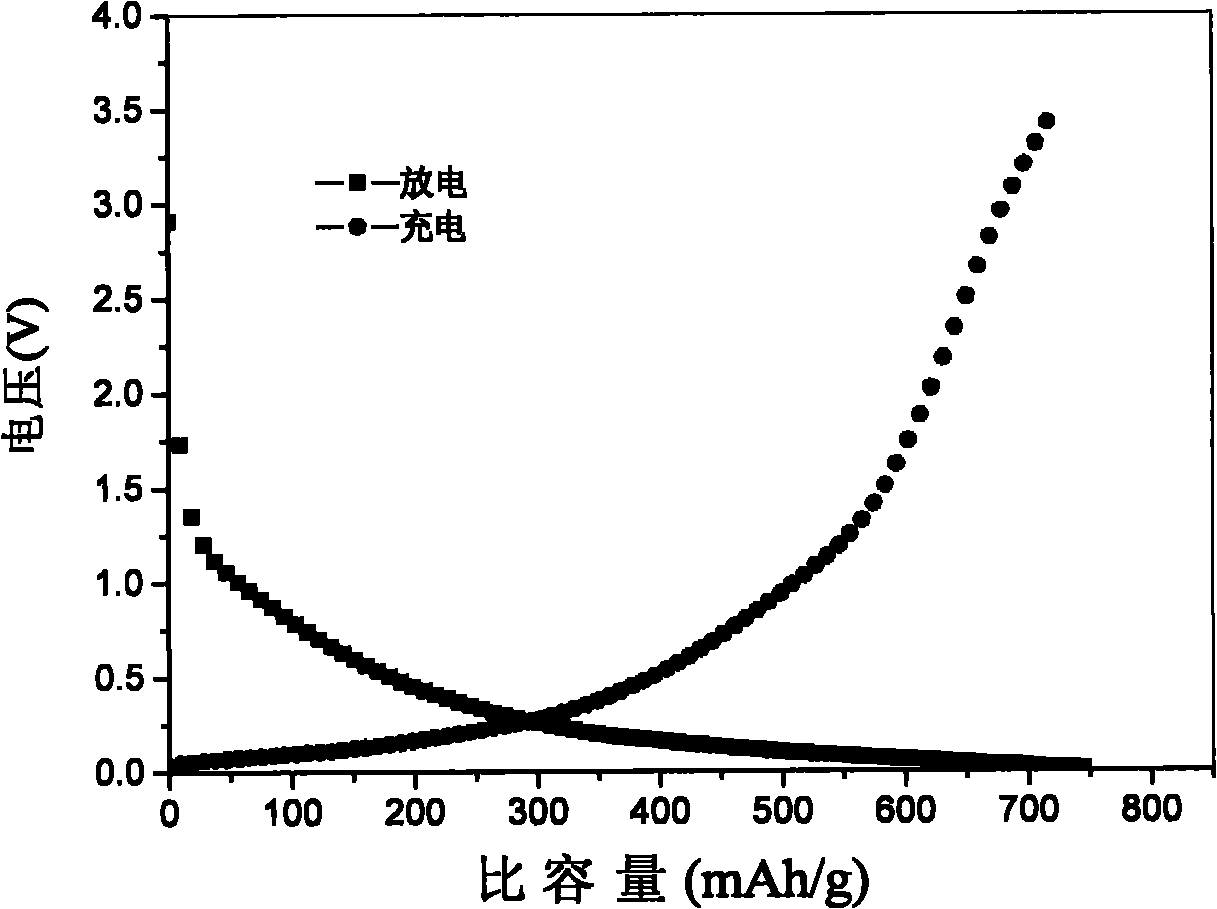
[0039] The artificial graphite is oxidized by the Hummer method to obtain graphite oxide, and then the graphite oxide is reduced with hydrazine hydrate to obtain reduced graphene. Put the prepared reduced graphene into a tube furnace with ammonia / helium / hydrazine mixed gas (ammonia 10%-50%; helium 90%-40%; hydrazine, 0-10%), 700 ℃ for 6 hours to obtain a nitrogen-doped graphene material. The prepared nitrogen-doped graphite material was mixed with conductive carbon black and polyvinylidene fluoride in N-methylpyrrolidone according to the mass ratio of 85:10:5, and evenly coated on the copper foil current collector, and vacuum-dried at 120°C. The negative pole piece was obtained, and then assembled into a coin cell in a glove box for testing. The capacity obtained by the test is 750mAh / g, and the charge-discharge curve diagram of the obtained material is shown in figure 1 .
Embodiment 2
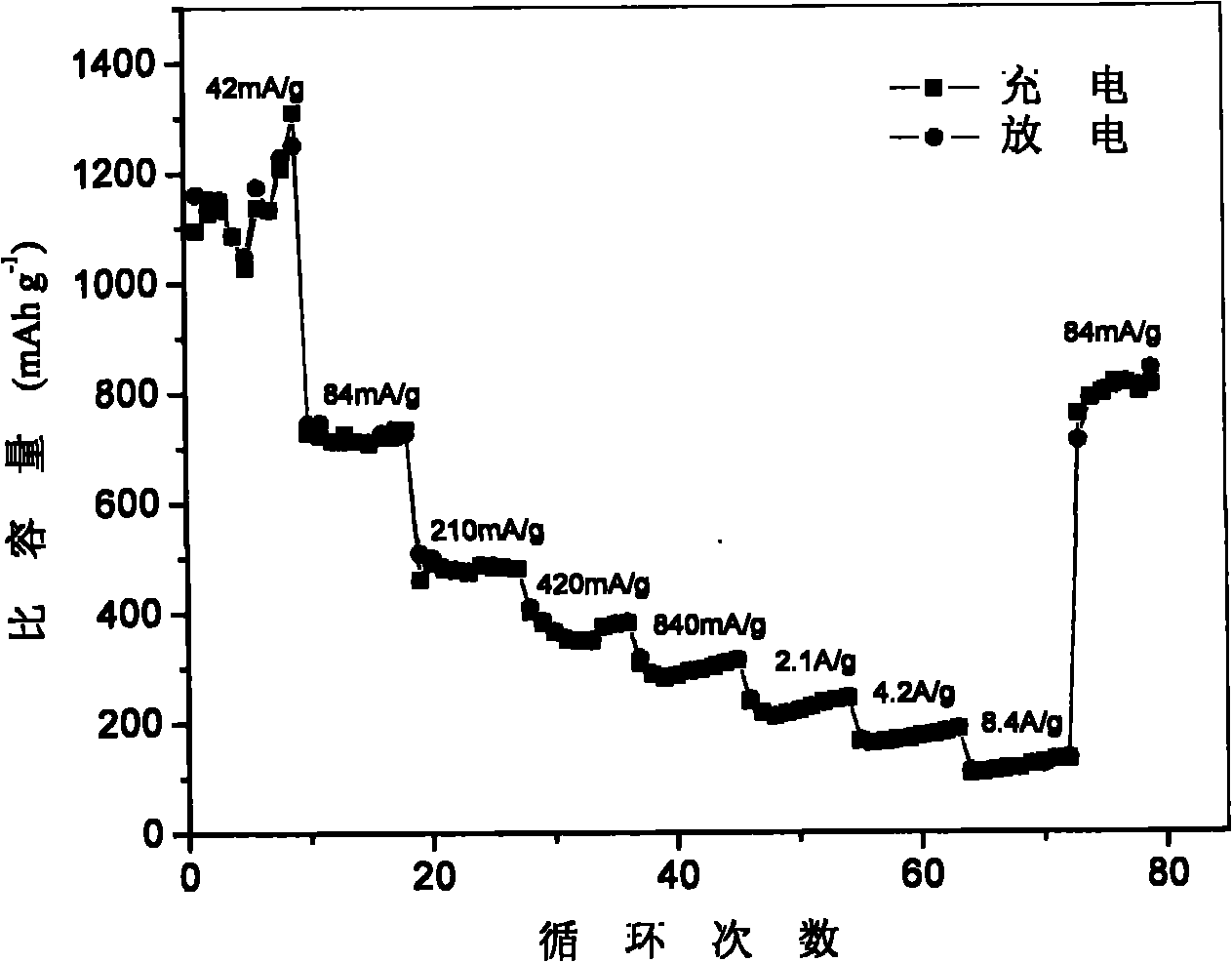
[0041] The graphene material prepared in Example 1 was put into a tube furnace with mixed gas of ammonia / acrylamine, and calcined at 800° C. for 2 hours to obtain a nitrogen-doped graphene material. The prepared nitrogen-doped graphene material was mixed with conductive carbon black and polyvinylidene fluoride in N-methylpyrrolidone according to the mass ratio of 85:10:5, and evenly coated on the copper foil current collector, and vacuum-dried at 120°C , to obtain the negative electrode sheet, and then assembled into a button battery in a glove box for testing. The graphene nitride prepared by the method has a specific capacity of 1150 mAh / g when the current density is 42 mA / g, and a specific capacity of 440 mAh / g when the current density is 210 mA / g. The rate performance and cycle performance graphs of the obtained materials are shown in figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com