G.japonicum mycelium polysaccharide refined substance freeze-dried powder injection and preparation method thereof
A technology of mycelium polysaccharides and freeze-dried powder injections, which is applied in the field of freeze-dried powder injections of refined polysaccharides from Zizhi mycelium and its preparation, achieving the effects of high polysaccharide content, reduced probability of adverse reactions, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
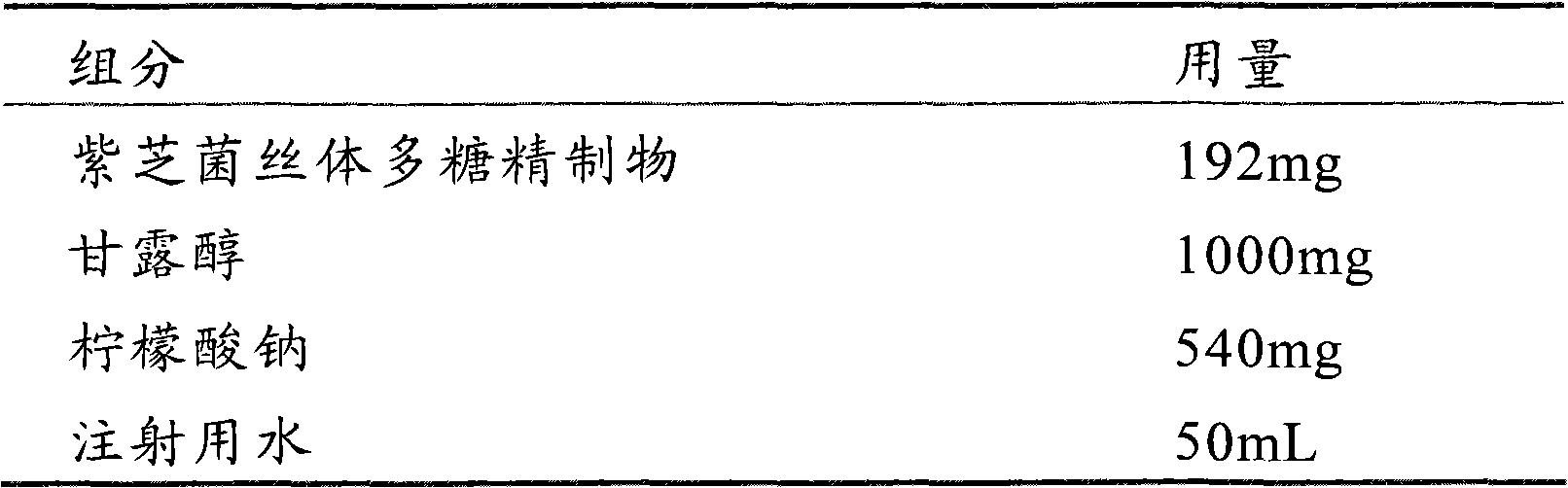
[0047] prescription composition
[0048]
[0049] Take the refined polysaccharide of Zizhi mycelia, dissolve it with an appropriate amount of water for injection, add mannitol and sodium citrate, stir for 20 minutes, add 0.8 mg of activated carbon for injection, heat at 80°C for 30 minutes, and filter it through a microporous membrane with a pore size of 0.22 μm after cooling Filter, pack in vials, 2mL per bottle, freeze-dry in a freeze-drying box, seal, and prepare injections. The pH of the obtained powder injection was 7.45.
Embodiment 2
[0051] prescription composition
[0052]
[0053] Take the refined polysaccharide of Zizhi mycelium, dissolve it with an appropriate amount of water for injection, add mannitol and sodium citrate, stir for 20 minutes, add 0.5% activated carbon for injection, heat at 80°C for 30 minutes, and filter it through a microporous membrane with a pore size of 0.22 μm after cooling Filter, pack in vials, 2mL per bottle, freeze-dry in a freeze-drying box, seal, and prepare injections. The pH of the obtained powder injection was 7.47.
Embodiment 3
[0055] prescription composition
[0056]
[0057] Take the refined polysaccharide of Zizhi mycelia, dissolve it with an appropriate amount of water for injection, add mannitol and sodium citrate, stir for 20 minutes, add 0.8 mg of activated carbon for injection, heat at 80°C for 30 minutes, and filter it through a microporous membrane with a pore size of 0.22 μm after cooling Filter, pack in vials, 2mL per bottle, freeze-dry in a freeze-drying box, seal, and prepare injections. The pH of the obtained powder injection was 7.46.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com