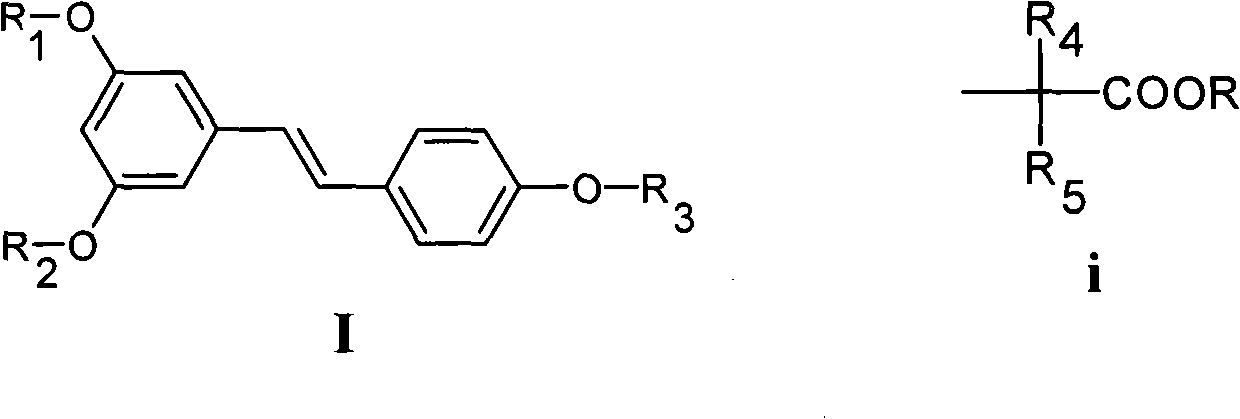
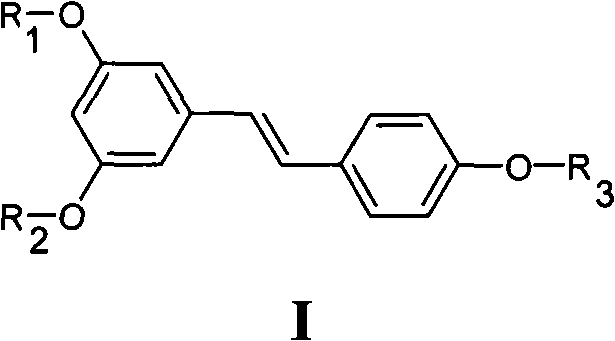
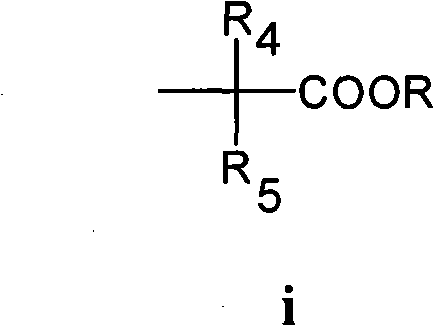
Resveratrol derivative and medical application thereof
A pharmacy and drug technology, applied in the field of resveratrol derivatives and their medical applications, can solve the problems of low oral bioavailability, biological activity to be improved, and poor water solubility of resveratrol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 3,5-dimethoxy-4'-(1-methyl-1-ethoxycarbonylethoxy)stilbene (I 1 )Synthesis
[0087] Add 10g resveratrol (43.8mmol), 18.3g K 2 CO 3 (131.4mmol), 100mL dry DMF, stirred and heated to 55°C under the protection of nitrogen, added 12.8mL (87.6mmol) of ethyl 2-bromoisobutyrate dropwise, after the addition was completed, continued heating reaction at 55°C for 16h, and stopped heating. After cooling, 100 mL of ethyl acetate was added to the reaction solution; the resulting solution was washed 3 times with 1N hydrochloric acid, 50 mL each time; then the organic layer was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and separate the residue by column chromatography with 200-300 mesh silica gel, and elute with a mixed solvent of dichloromethane: ethyl acetate: acetic acid (9.5: 0.5: 0.2) to obtain 4'-(1-methanol (1-ethoxycarbonylethoxy)stilbene as a white solid, yield 78%.
[0088] Dissolve 1.2 g of 4′-(1-methyl-1-ethoxycarbonylethoxy) stilbene in 20 ...
Embodiment 2
[0089] Example 2 3,5-dimethoxy-4'-(1-methyl-1-carboxyethoxy)stilbene (I 2 )Synthesis
[0090] Will I 1 0.39g (1.06mmol) was dissolved in 20mL of MeOH / H 2 O (1:1), add 0.46g of K 2 CO 3 (3.36mmol), heat to reflux for 12h, stop heating, after cooling, add 40mL of water, adjust the pH to 3-4 with hydrochloric acid; then extract 3 times with ethyl acetate (20mL / time), combine the extracts, wash with water until Neutral, anhydrous Na for organic layer 2 SO 4 Dry, filter, concentrate and separate by column chromatography with 200-300 mesh silica gel, and elute with a mixed solvent of dichloromethane: ethyl acetate: acetic acid (7:3:0.08) to obtain the target compound I 2 , yield 89%. 1 H-NMR (d 6 -DMSO): δppm 1.10-1.12 (6H, s), 3.77 (6H, s), 6.38-6.39 (1H, m), 6.73-6.74 (2H, br.s), 6.92-6.94 (2H, d, J = 8.8Hz), 6.99-7.03 (1H, d, J = 16.4Hz), 7.18-7.22 (1H, d, J = 16.8Hz), 7.50-7.52 (2H, d, J = 8.8Hz).
[0091] Using conventional reaction conditions, the target compound I...
Embodiment 3
[0092] Example 3 3-(1-methyl-1-ethoxycarbonylethoxy)-4'-methoxystilbene (I 3 ) and 3,5-bis(1-methyl-1-ethoxycarbonylethoxy)-4'-methoxystilbene (I 4 )Synthesis
[0093] Dissolve resveratrol 10g (43.8mmol) in 200mL acetone, add 15.13g of K 2 CO 3(109.5mmol), 3.33mL of CH 3 I (65.7mmol), heated under the protection of nitrogen for reflux reaction for 10h, stop heating, after cooling, filter, wash the filter cake with acetone, combine the filtrate and lotion, concentrate and separate through column chromatography with 200-300 mesh silica gel, and use petroleum Ether: ethyl acetate: acetic acid (8.5: 1.5: 0.16) mixed solvent was eluted to obtain 8.9g 4'-methoxystilbene (II 1 ) and 2.5g 3,4'-dimethoxystilbene (II 2 ). II 1 H NMR spectrum ( 1 H-NMR (d 6 -DMSO) data are as follows: δppm 3.76 (3H, s), 6.13 (1H, br.s), 6.39 (2H, br.s), 6.80 (2H, d, J=8.8Hz), 6.90 (1H, d, J = 16.4Hz), 6.97 (1H, d, J = 16.4Hz), 7.48 (2H, d, J = 8.8Hz), 9.24 (2H, s). II 2 H NMR spectrum ( 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com