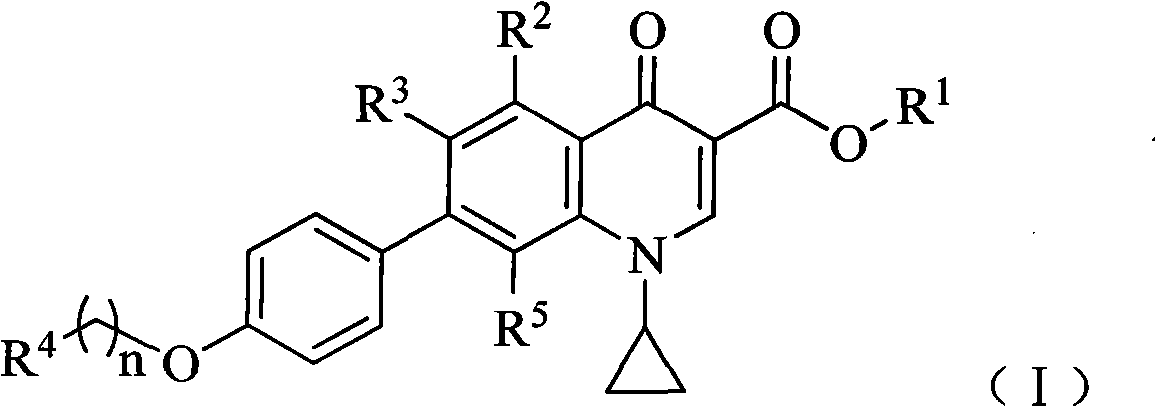
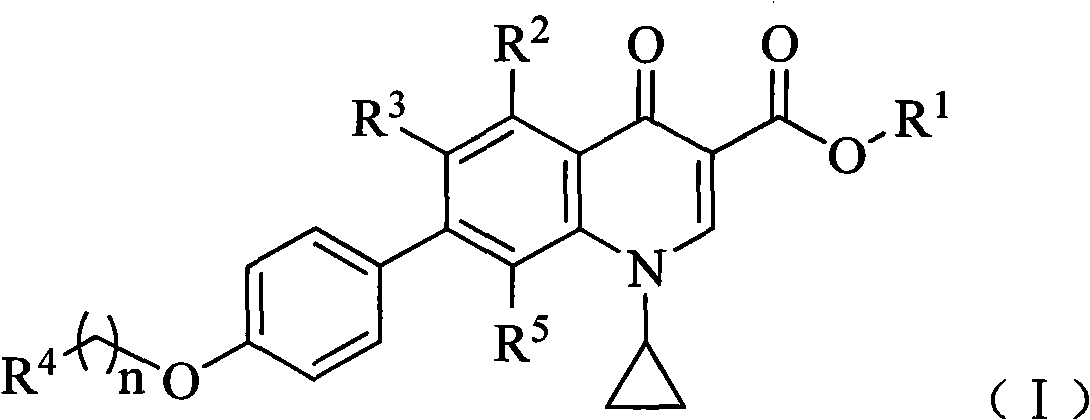
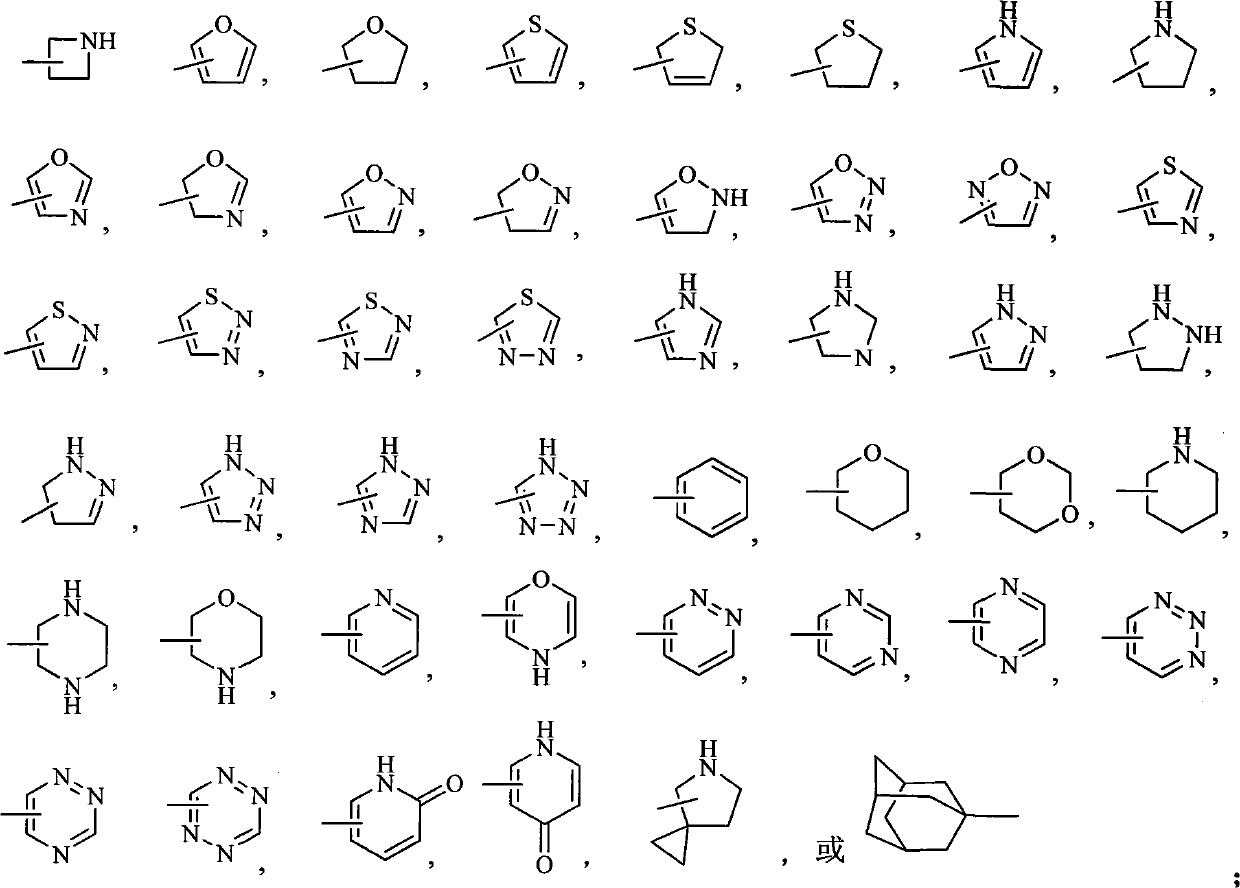
7-phenyl quinolone compounds
A compound and solvate technology, applied in the field of medicine, can solve the problems of unsatisfactory antibacterial effect of Gram-positive bacteria and unsatisfactory safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 7-[4-[2-(1H-pyrazol-1-yl)ethoxy]phenyl]-1-cyclopropyl-8-(difluoromethoxy)-4-oxo- Preparation of 1,4-dihydroquinoline-3-carboxylic acid (compound 1)
[0136]
[0137] 7-Bromo-1-cyclopropyl-8-(difluoromethoxy)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester 400mg (1mmol) was added to a dry reaction flask , 15mL toluene, stir to dissolve. Then 1-[2-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy]ethyl]-1H-pyridine was added Azole 314.2 mg (1 mmol), 0.5 mL of 2N sodium carbonate solution, Pd (PPh 3 ) 4 Cl 2 28mg. The reaction solution was stirred and refluxed under nitrogen atmosphere. After completion of the reaction of the raw materials, the reaction mixture was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated to dryness. Purified by column chromatography to obtain 0.36 g of solid product, yield: 71.4%.
[0138] 0.15 g (0.3 mmol) of 7-[4-[2-(1H-pyrazol-1-yl)ethoxy]phenyl]-1-cyclopropyl-8-(difluoromet...
Embodiment 2
[0141] Example 2 1-Cyclopropyl-8-(difluoromethoxy)-4-oxo-7-[4-[2-(pyrrolidin-1-yl)ethoxy]phenyl]-1, Preparation of 4-dihydroquinoline-3-carboxylic acid (compound 2)
[0142]
[0143] Under nitrogen protection, add 7-bromo-1-cyclopropyl-8-(difluoromethoxy)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester to the dry reaction flask 400 mg (1 mmol), 20 mL dioxane, 4-[2-(pyrrolidin-1-yl)ethoxy]phenylboronic acid 235 mg (1 mmol), Na 2 CO 3 106mg (1mmol) and catalyst Pd (PPh 3 ) 4 50 mg was stirred to dissolve and the reaction was stirred overnight at 100°C under nitrogen atmosphere. After the reaction was completed, a mixed solution of ethyl acetate and water (1:1) was added to the reaction system, and the organic layer was separated. The organic layer was dried with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated to dryness, and purified on a preparative chromatography column ( n-hexane:ethyl acetate=1:1) to obtain 307 mg of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com