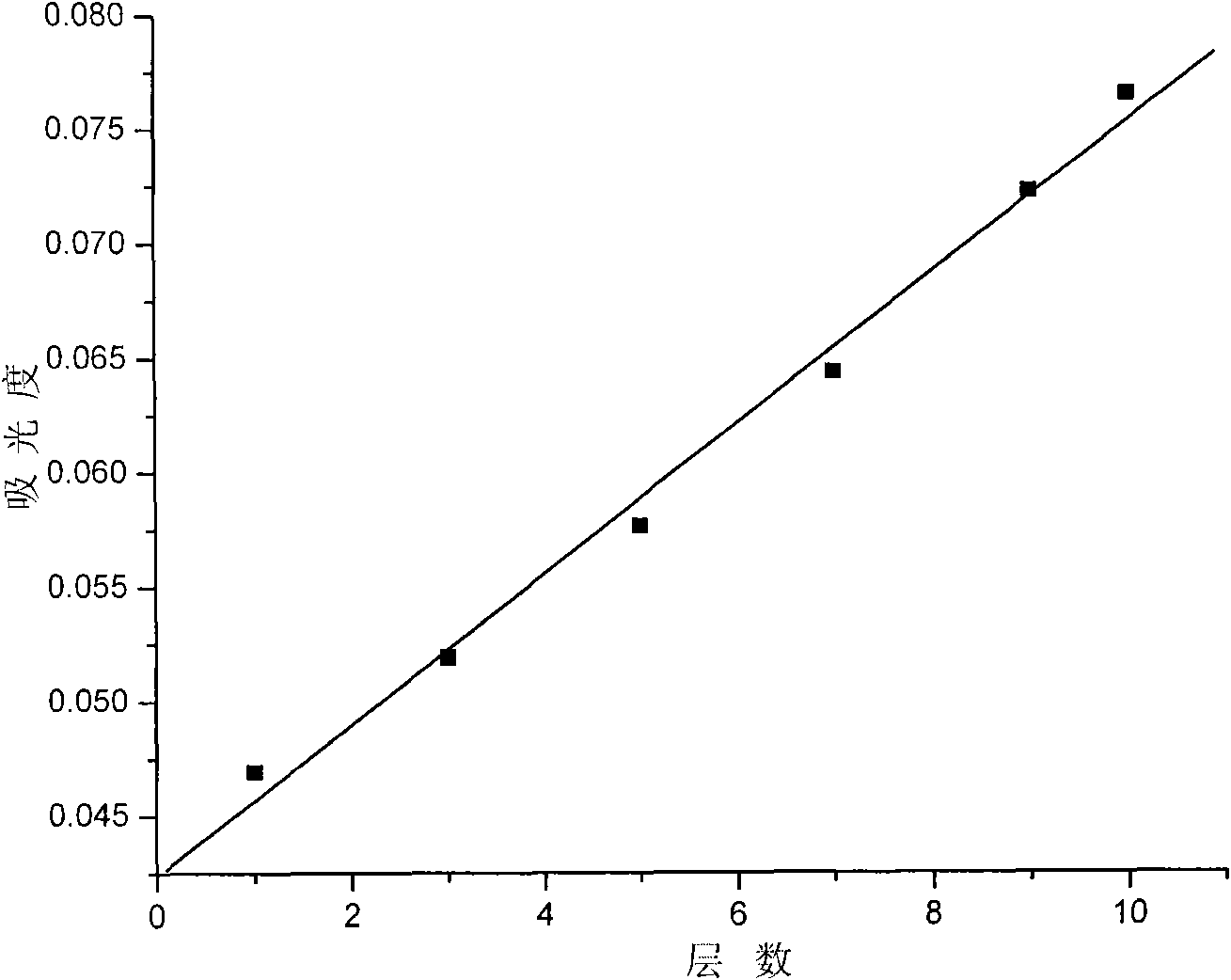Hydrogen peroxide electrochemical sensor and manufacturing method thereof
A chemical sensor, hydrogen peroxide technology, applied in the direction of material electrochemical variables, etc., can solve the problems of poor chemical stability and thermal stability of enzyme molecules, difficult electron transfer, and difficult exposure, etc., to achieve good response, rapid electrocatalytic performance, electrocatalytic The effect of chemical signal enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. The preparation mass fraction is the naphthol green solution A of 1g / L;
[0029] 2. Preparation of nitrate hydrotalcite by ion exchange method:
[0030] a. Add 0.01mol of solid Co(NO 3 ) 2 ·6H 2 O and 0.005 mol of solid Al(NO 3 ) 3 9H 2 O and 0.06mol urea were dissolved in 50mL of deionized water, and crystallized in a 90ml polytetrafluoroethylene pressure reaction vessel at 120°C for 48 hours, then centrifugally washed with deionized water until the pH was about 7,50 Dry at ℃ for 24 hours to obtain carbonate intercalated hydrotalcite powder;
[0031] b. Take 0.3 g of the above-mentioned carbonate intercalation hydrotalcite powder and solid NaNO 3 63.75g dissolved in 300mL to remove CO 2 After uniform dispersion in deionized water, add 0.09mL of concentrated nitric acid and stir at 20°C under a nitrogen atmosphere to carry out ion exchange reaction for 24 hours and then use 2 Centrifugal washing with deionized hot water until the pH is about 7, and vacuum dr...
Embodiment 2
[0037] 1. The preparation mass fraction is the naphthol green solution A of 1g / L;
[0038] 2. With embodiment 1;
[0039] 3. Obtain clear transparent colloidal solution B with embodiment 1;;
[0040] 3. The glassy carbon electrode sheet is made of 0.05μm Al 2 o 3 The powder is ground into a mirror surface, cleaned with ultrapure water, ultrasonicated in concentrated nitric acid, absolute ethanol, and ultrapure water for 30 seconds, and the cleaned glassy carbon electrode sheet is soaked in solution A for 10 minutes, and fully cleaned with deionized water Finally, placed in solution B, soaked for 10 minutes and fully cleaned to obtain a cycle of naphthol green and hydrotalcite composite film;
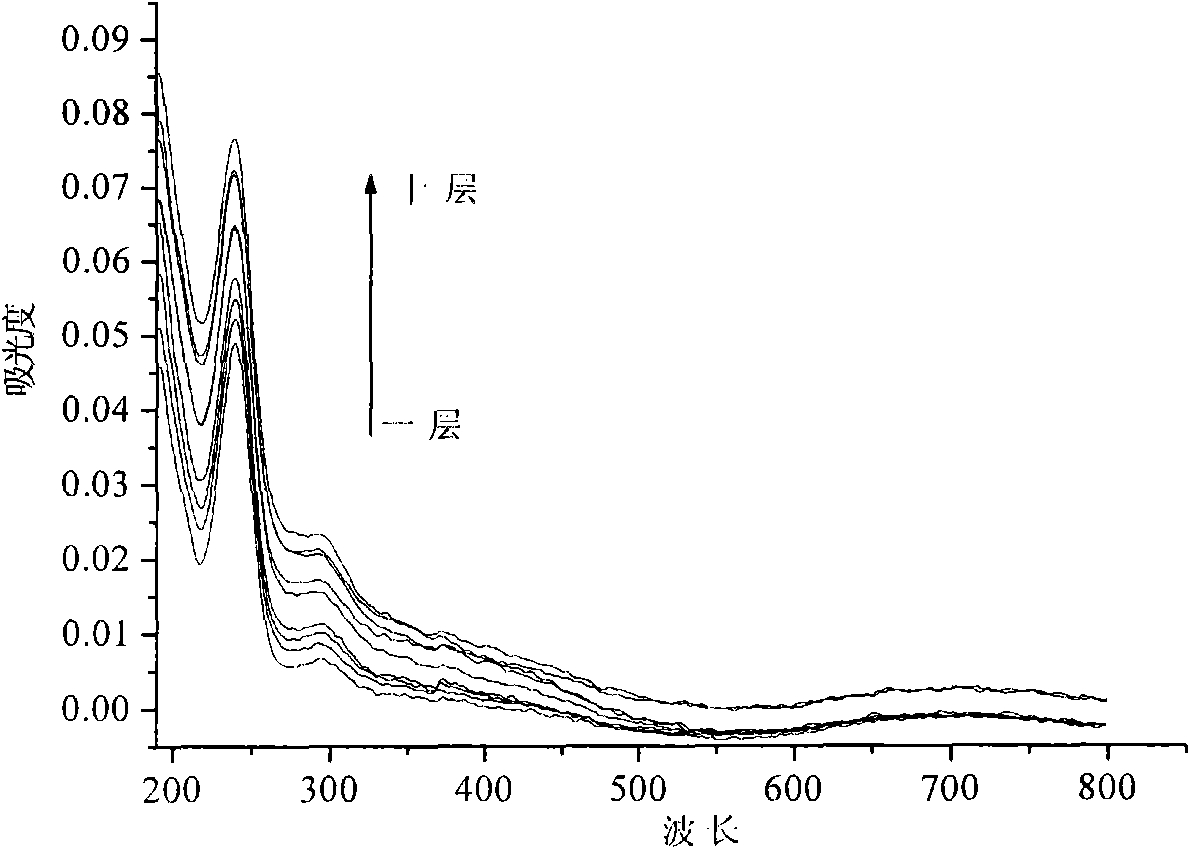
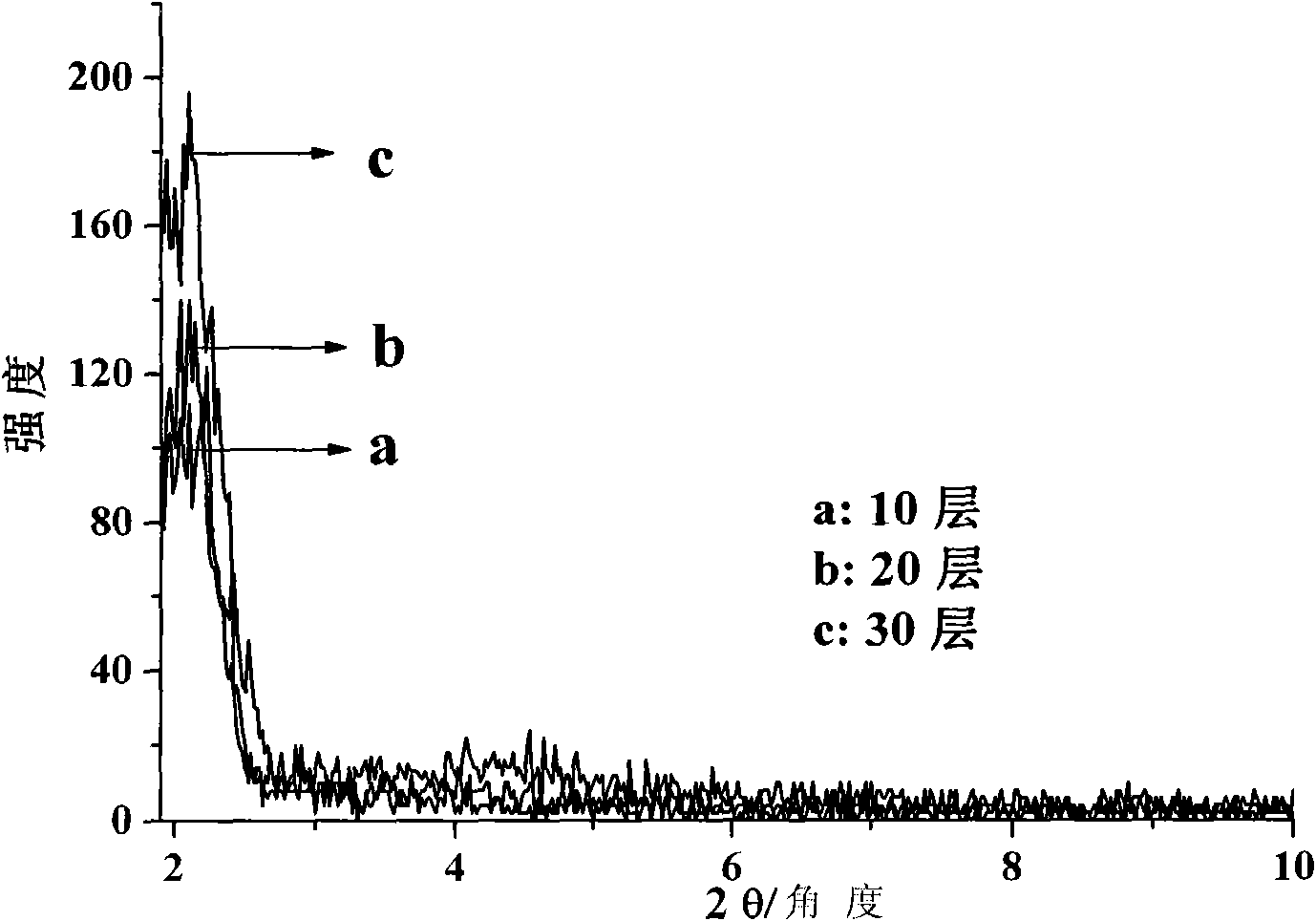
[0041] 4. Repeat steps 3 and 12 times to obtain a multilayer composite film of naphthol green and hydrotalcite, and vacuum-dry to obtain a hydrogen peroxide electrochemical sensor.
[0042] Utilize the electrochemical workstation with silver / silver chloride as the reference electrode...
Embodiment 3
[0044] 1. The preparation mass fraction is the naphthol green solution A of 2g / L;
[0045] 2. With embodiment 1;
[0046] 3. Obtain clear transparent colloidal solution B with embodiment 1;;
[0047] 3. The glassy carbon electrode sheet is made of 0.04μm Al 2 o 3 The powder is ground into a mirror surface, cleaned with ultrapure water, ultrasonicated in concentrated nitric acid, absolute ethanol, and ultrapure water for 20 seconds, and the cleaned glassy carbon electrode sheet is soaked in solution A for 12 minutes, and fully cleaned with deionized water Finally, placed in solution B, soaked for 12 minutes and fully cleaned to obtain a cycle of naphthol green and hydrotalcite composite film;
[0048] 4. Repeat steps 3 and 15 times to obtain a multi-layer composite film of naphthol green and hydrotalcite, and vacuum-dry to obtain a hydrogen peroxide electrochemical sensor.
[0049] Use silver / silver chloride as a reference electrode, platinum wire as a counter electrode, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com