Puerarin derivatives containing nitrate substituent groups and preparation method and medicinal application thereof
A technology of puerarin derivatives and substituents, which is applied in the field of chemical pharmaceuticals and can solve problems such as instability and failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
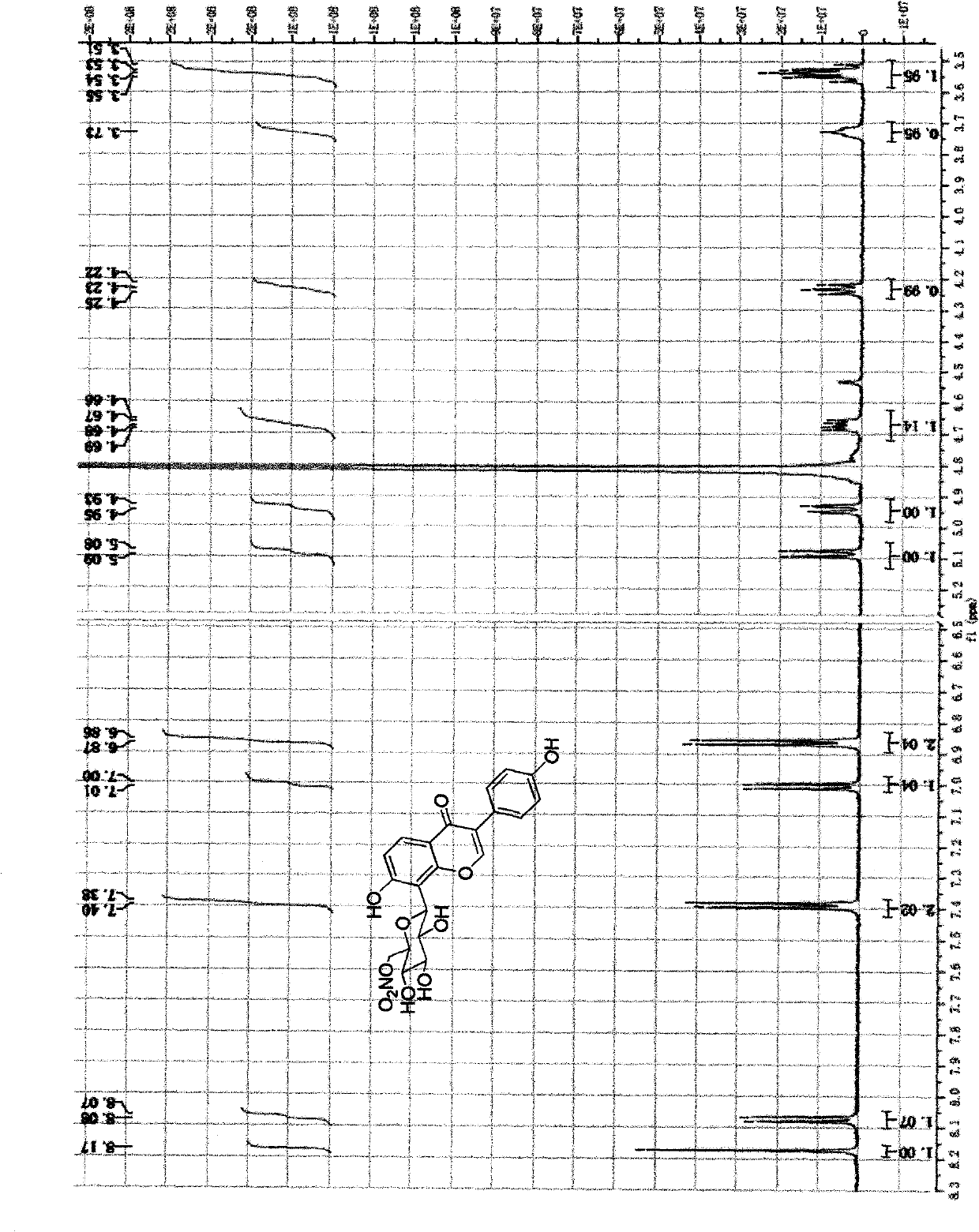
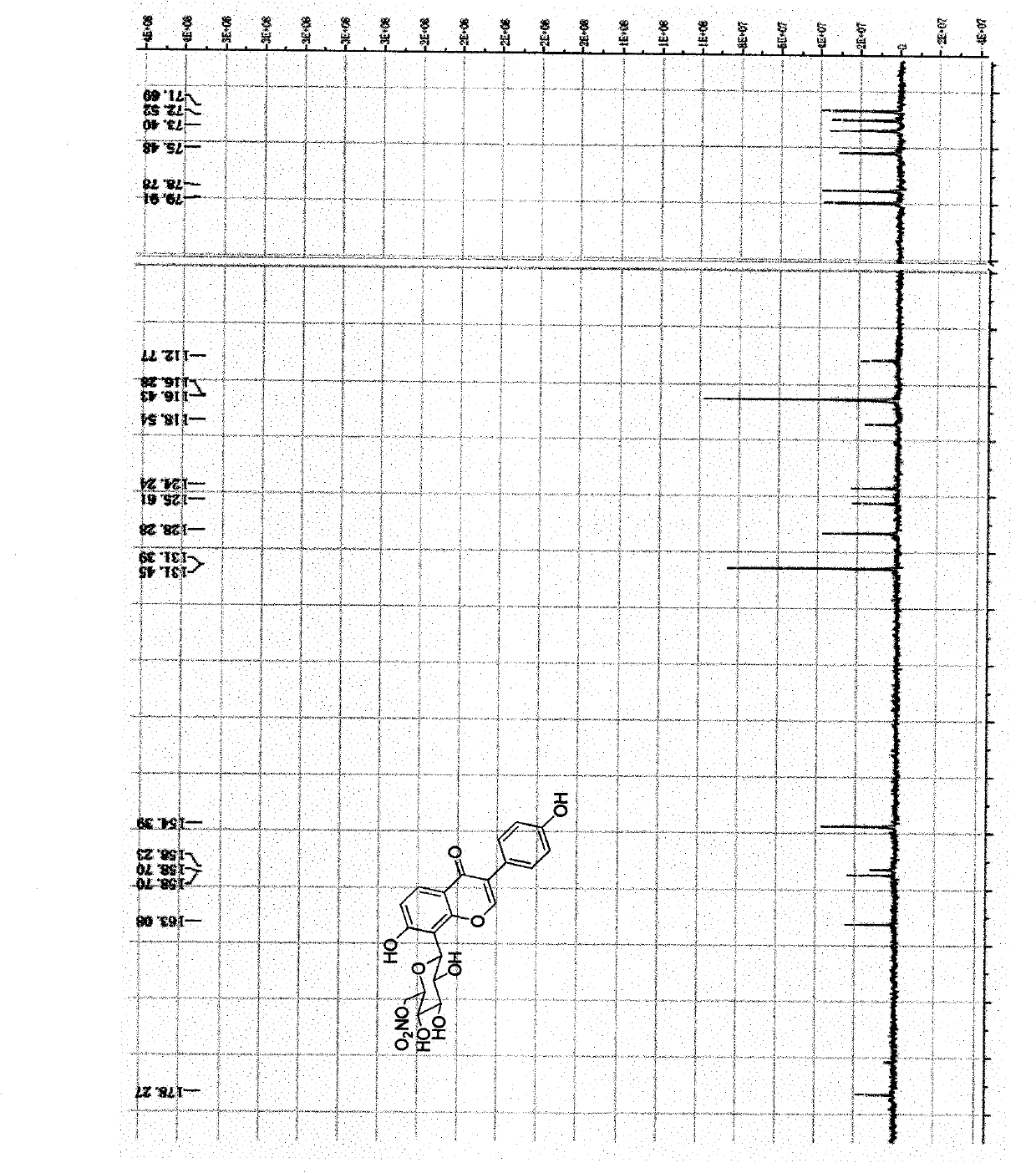
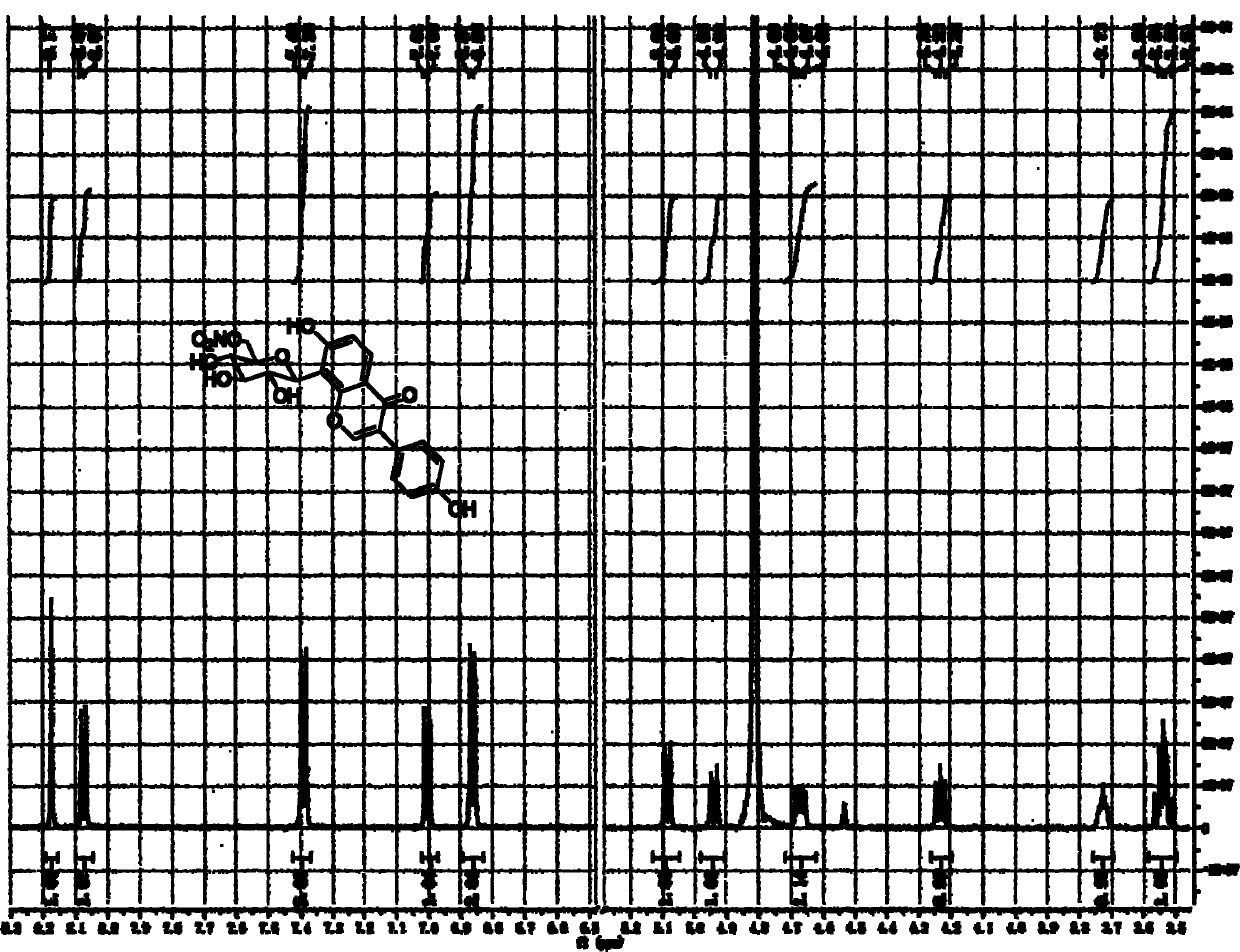
Image
Examples
Embodiment Construction
[0061] The method for chemically synthesizing above-mentioned nitrate derivatives provided by the invention is shown in the following synthetic route:
[0062]
[0063] 1) Methylsulfonylation of the 6-position hydroxyl group of puerarin (P->Ms-P);
[0064] Dissolve 2.49g (6mmol) puerarin in 15ml pyridine, put it in a 100ml pear-shaped bottle, connect a 60ml constant pressure dropping funnel, and fill the funnel with 30ml pyridine and 0.71g (6.2mmol) methanesulfonyl chloride. The liquid was dripped under constant pressure at 0 degrees Celsius, and the dripping was completed for about 2 hours, and the stirring was continued overnight, and the temperature was gradually increased from 0 degrees Celsius to room temperature. The supernatant was diluted and developed by TLC (developing solvent: ethyl acetate: methanol: acetic acid = 20: 1: 0.5), the main product Rf = 0.4. After the mixture was filtered, the solvent was evaporated and then separated by column chromatography with m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com