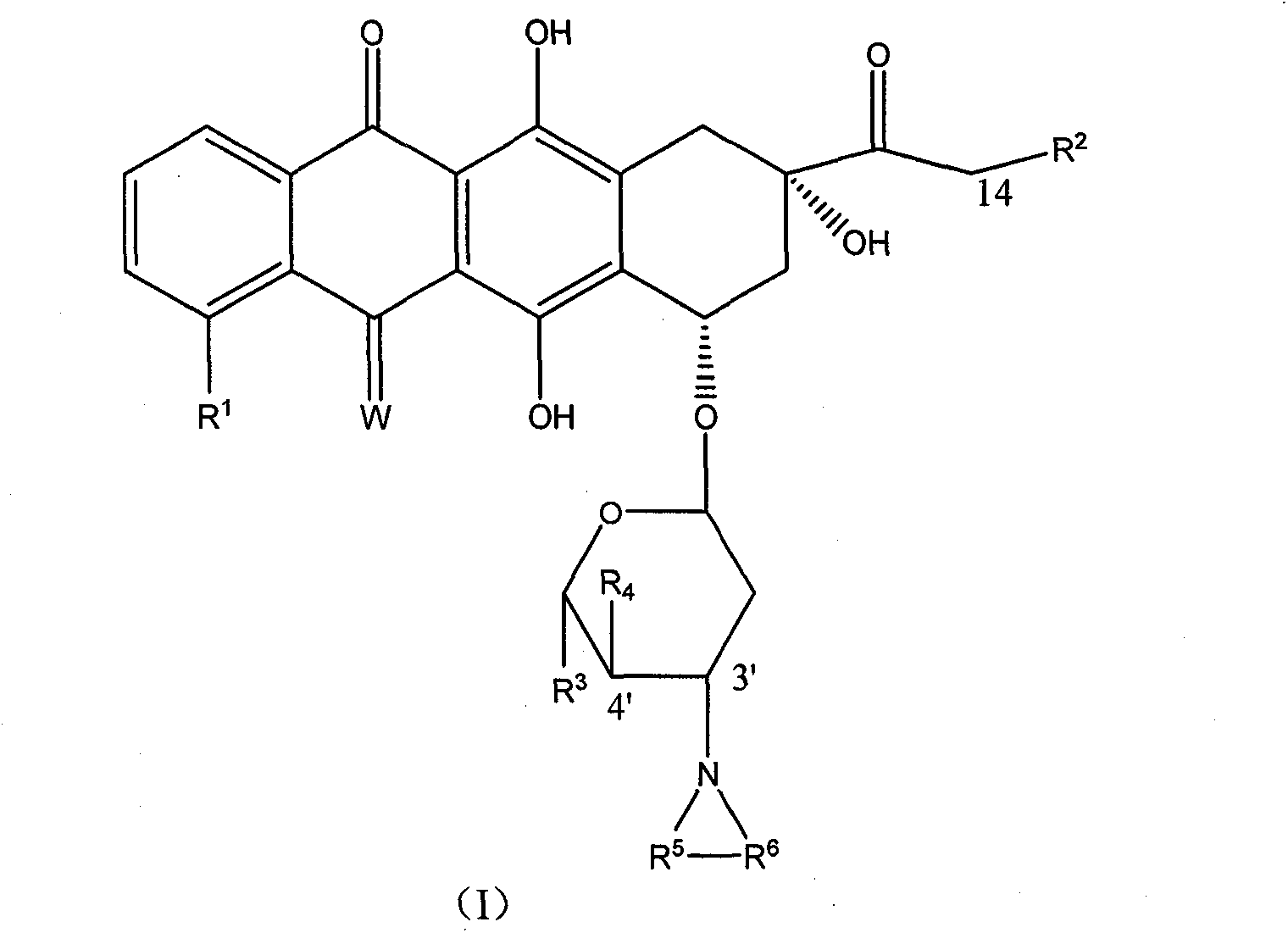
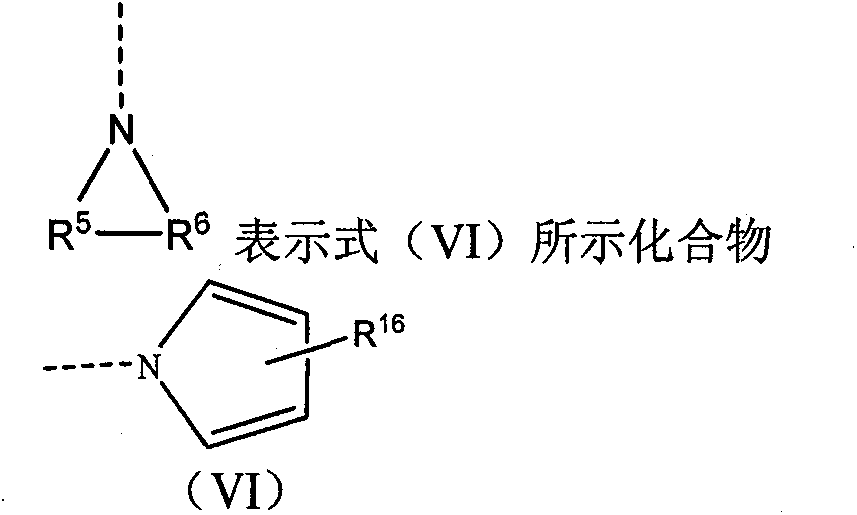
High-activity epirubicin derivative, preparation method thereof and application thereof
A preparation and compound technology, applied in the field of derivatives of epirubicin, can solve the problems of heart failure even a few weeks after the termination of treatment, and may be ineffective to the corresponding drug treatment, increased cardiotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0117] Embodiment 13'-pyrrolyl epirubicin
[0118] Add 3.076 g of epirubicin (Epirubicin) hydrochloride, 300 ml of distilled water, 300 ml of 1,2-dichloroethane, 30 ml of 2,5-dimethoxytetrahydrofuran and 6 ml of glacial acetic acid into a 1 L three-neck flask. Under the protection of argon, it was heated to reflux for 45 minutes, and the reaction was completed. The reaction solution was cooled to room temperature, poured into 200ml of ice water, and allowed to stand for liquid separation. The organic phase was washed once with 200ml of saturated brine, dried over anhydrous magnesium sulfate, filtered and spin-dried; the aqueous phase was stirred in an ice bath by adding 100ml of 5% aqueous sodium bicarbonate solution, extracted with chloroform (50ml×3), combined the chloroform layers, and the chloroform layers Wash once with 100 ml of saturated brine, filter and spin off the solvent, combine the obtained crude product with the above obtained crude product, and purify by colum...
Embodiment 23
[0119] Embodiment 23'-pyrrolyl epirubicin
[0120] Add 3.076 g of epirubicin (Epirubicin) hydrochloride, 500 ml of distilled water, 30 ml of 2,5-dimethoxytetrahydrofuran, 6 ml of glacial acetic acid and 435 mg of sodium acetate into a 1 L three-necked flask. Under the protection of argon, the reaction was stirred at 50° C. for 4 hrs, and the reaction was completed. The reaction solution was cooled to room temperature, and suction filtered to obtain a filter cake. The target compound was obtained by recrystallization of the filter cake. MS: 592 (M-1)
Embodiment 33
[0121] Example 3 3'-pyrrolyl-4'-(pyran-2-yl) epirubicin MS: 677
[0122] With the method in embodiment 1 or embodiment 2, prepare with 4'-(pyran-2-yl) epirubicin as raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com