Finasteride-containing orally disintegrating tablets and preparation method thereof
A technology of oral disintegrating tablets and finasteride, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as delaying the effective treatment time of patients, and achieve good taste , good promotion prospects, avoid the effect of repeated investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
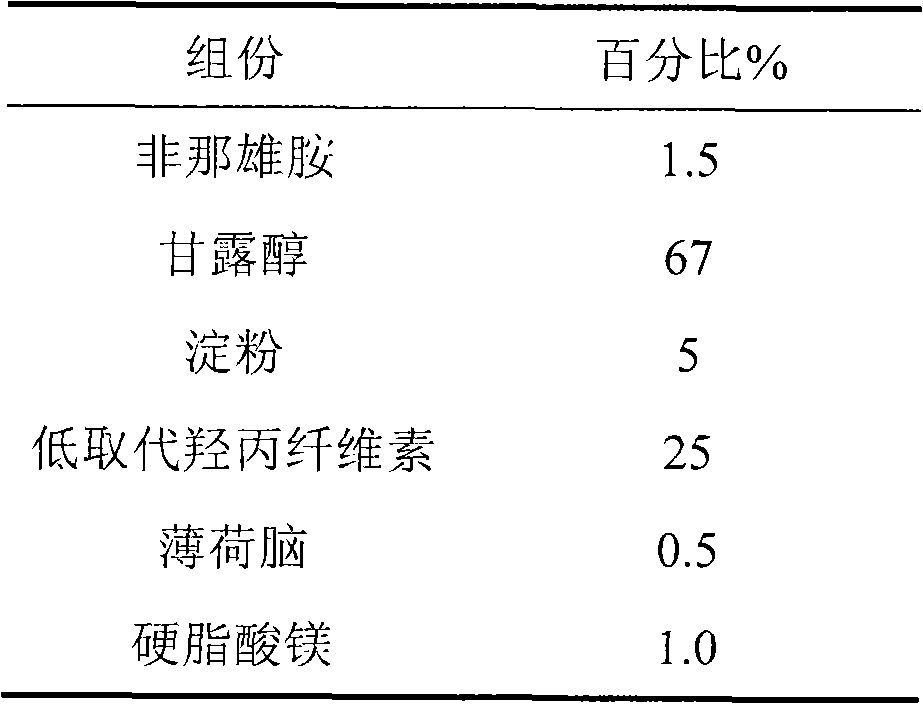
[0013] The formulation of the present invention consists of the following components in parts by weight
[0014]
[0015] The dosage form can be produced using conventional tablet pharmaceutical equipment and prepared using a compression process, and the specific preparation method is as follows:
[0016] 1. Weigh the prescribed amount of finasteride and mannitol, and pulverize them together through a pulverizer until the powder D90 is less than or equal to 75 microns;
[0017] 2. Add low-substituted hydroxypropyl cellulose, increase in equal amounts, and mix well;
[0018] 3. Granulate with starch slurry as binder;
[0019] 4. Add the prescribed amount of menthol and magnesium stearate after blast drying, and mix well;
[0020] 5. Carry out intermediate content detection and tablet compression to obtain orally disintegrating tablets.
Embodiment 2
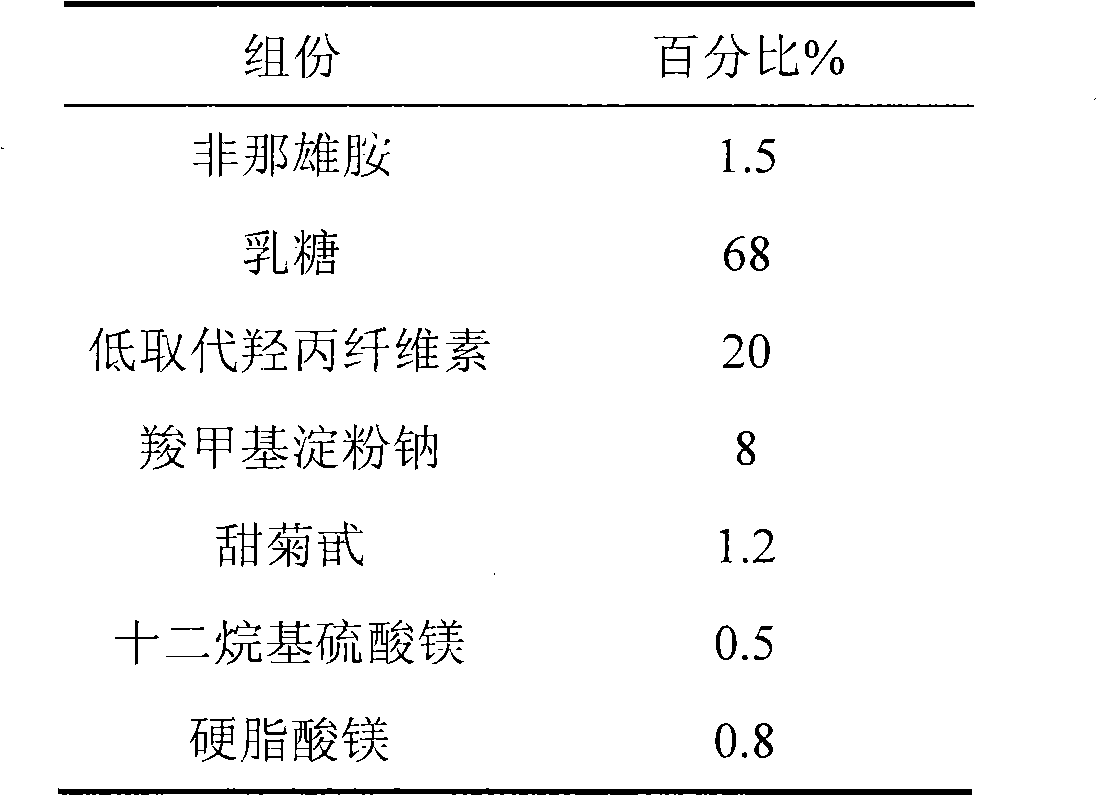
[0022] The formulation of the present invention consists of the following components in parts by weight
[0023]
[0024] The dosage form can be produced using conventional tablet pharmaceutical equipment and prepared using a compression process, and the specific preparation method is as follows:
[0025] 1. Weigh the prescribed amount of finasteride and lactose, and pulverize them together through a pulverizer until the powder D90 is less than or equal to 50 microns;
[0026] 2. Then add sodium carboxymethyl starch and low-substituted hydroxypropyl cellulose, add in equal amounts, and mix well;
[0027] 3. Granulate with water as binder;
[0028] 4. Add stevioside, lauryl magnesium sulfate, and magnesium stearate in the prescribed amount after blast drying, and mix well;
[0029] 5. Carry out intermediate content detection and tablet compression to obtain orally disintegrating tablets.
Embodiment 3
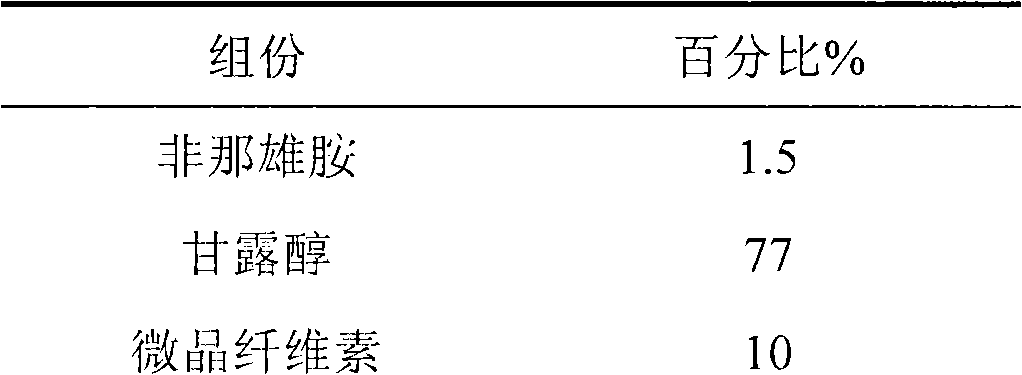
[0031] The formulation of the present invention consists of the following components in parts by weight
[0032]
[0033]
[0034] The dosage form can be produced using conventional tablet pharmaceutical equipment and prepared using a compression process, and the specific preparation method is as follows:
[0035] 1. Weigh the prescribed amount of finasteride and mannitol, and use a pulverizer to pulverize them until the powder D90 is less than or equal to 20 microns;
[0036] 2. Add low-substituted hydroxypropyl cellulose and microcrystalline cellulose, increase in equal amounts, and mix well;
[0037] 3. Granulate with water;
[0038] 4. Add the prescribed amount of stevioside and magnesium stearate after blast drying, and mix well;
[0039] 5. Carry out intermediate content detection and tablet compression to obtain orally disintegrating tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com