Medicament compound adopting bicyclo-ethanol as active component and preparation thereof
A technology of active ingredients and compositions, which is applied in the field of pharmaceutical compositions and preparations with bicyclol as the active ingredient, can solve the problems of no solid precipitation of bicyclol, improve bioavailability, improve clinical curative effect, enhance absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] [Prescription composition]
[0074] Bicyclic alcohol 40mg
[0075] LABRAFIL M 1944CS 200mg
[0076] Cremophor RH40 300mg
[0077] Tween-80 300mg
[0078] PEG400 200mg
[0079] Propyl gallate (antioxidant) 0.01%
[0080] [Preparation method] According to the prescription, weigh the auxiliary materials, heat to 60-70°C and mix uniformly; add the prescription amount of bicyclic alcohol, stir and dissolve at 60-70°C to obtain a uniform solution, and get it.
[0081] [Emulsification performance inspection] Method: take the drug solution, add about 20 times 37℃ water, shake or stir, observe the emulsification and dispersion of the drug solution in the water; and use the Nicomp laser particle size analyzer to measure the particle size distribution.
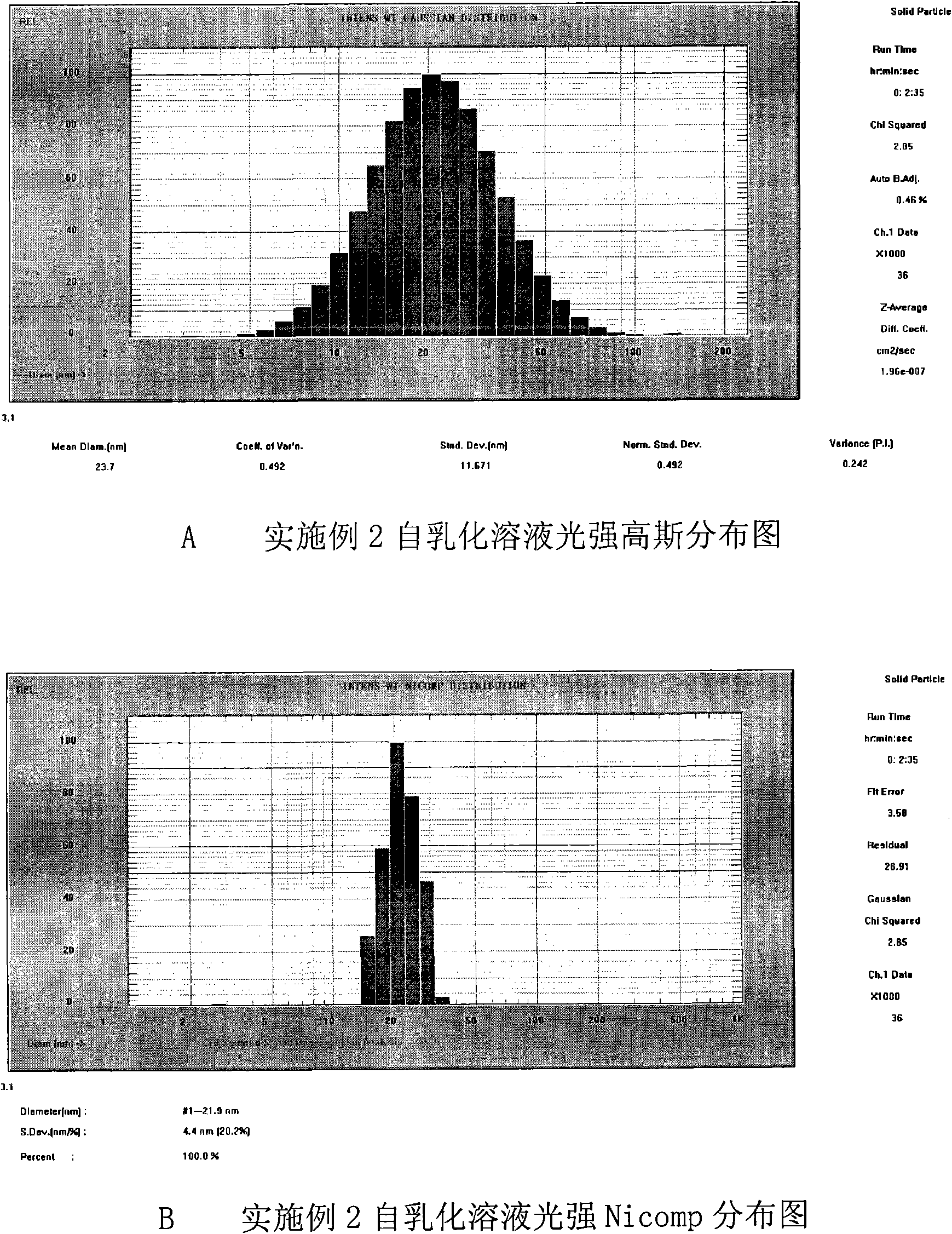
[0082] Result: The pharmaceutical composition showed a light blue transparent microemulsion after adding water, with a particle size distribution range of 20nm-50nm. See attached for particle size distribution figure 1 .
[0083] [Bioavailabil...
Embodiment 2
[0088] [Prescription composition]
[0089] Bicyclic alcohol 40mg
[0090] LABRAFIL M 1944CS 200mg
[0091] Cremophor RH40 400mg
[0092] Tween-80 200mg
[0093] PEG400 100mg
[0094] Propyl gallate (antioxidant) 0.01%
[0095] [Preparation method] Same as Example 1.
[0096] [Emulsification performance inspection] Same as Example 1.
[0097] Result: The pharmaceutical composition showed a light blue transparent microemulsion after adding water, with a particle size distribution range of 20nm-50nm. See attached for particle size distribution image 3 .
Embodiment 3
[0099] [Prescription composition]
[0100] Bicyclic alcohol 40mg
[0101] Tween-80 600mg
[0102] PEG400 200mg
[0103] [Preparation method] Same as Example 1.
[0104] [Emulsification performance inspection] Same as Example 1.
[0105] Results: The pharmaceutical composition became a transparent solution after adding water.
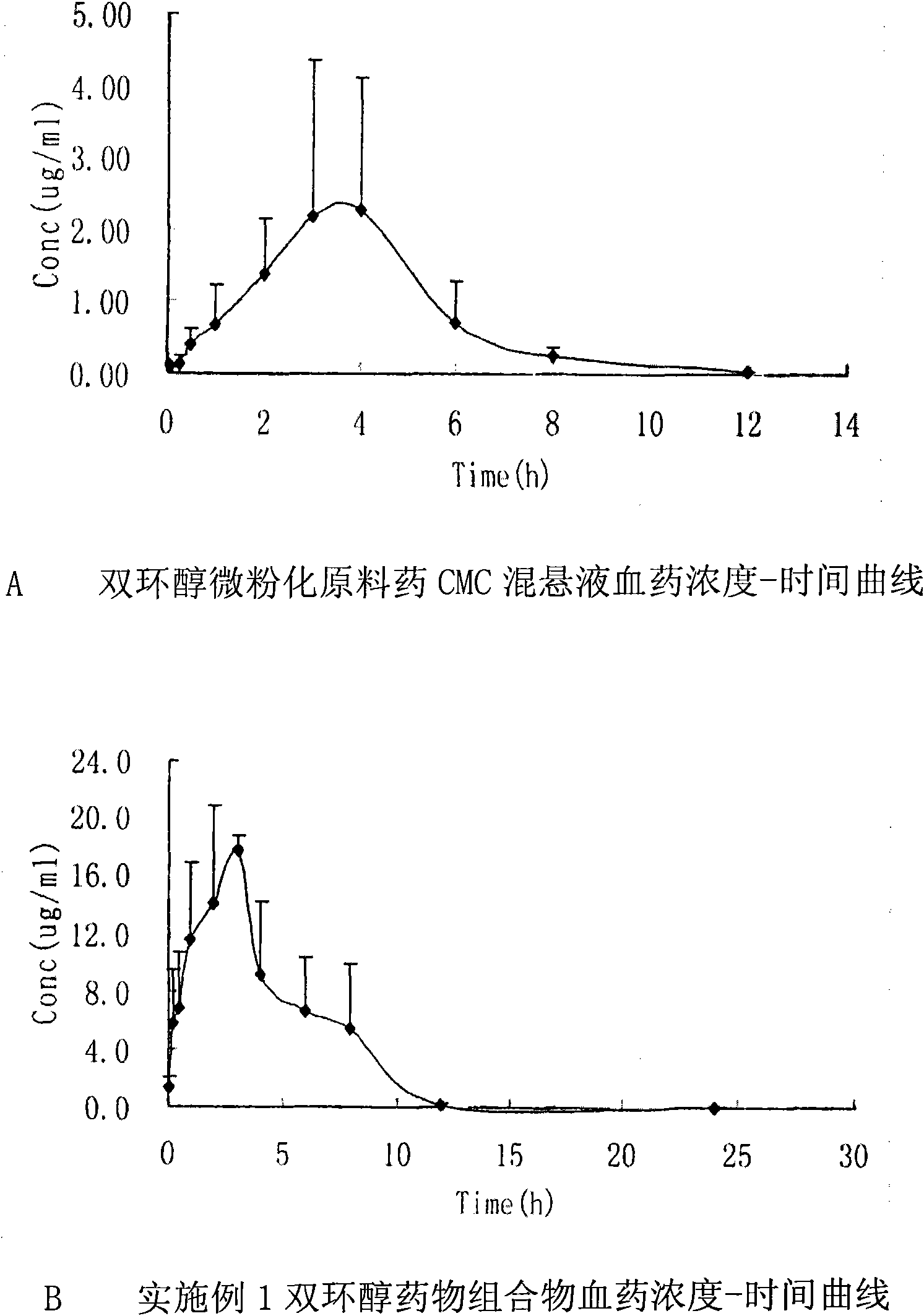
[0106] [Bioavailability experiment] Test purpose: To compare the in vivo pharmacokinetic characteristics of the bicyclic alcohol pharmaceutical composition in Example 3 and the CMC suspension of the micronized bicyclic alcohol raw material drug after oral administration in rats.
[0107] Methods: SD rats were given a single intragastric administration ①The bicyclic alcohol pharmaceutical composition in Example 3 ②The micronized bicyclic alcohol crude drug CMC suspension 100mg / kg, 5min, 15min, 30min, 1h, 2h, 3h after administration , 4h, 6h, 8h, 12h, 24 each time point from the eye canthus vein to take blood, HPLC blood sample determination, draw blood concentration-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com