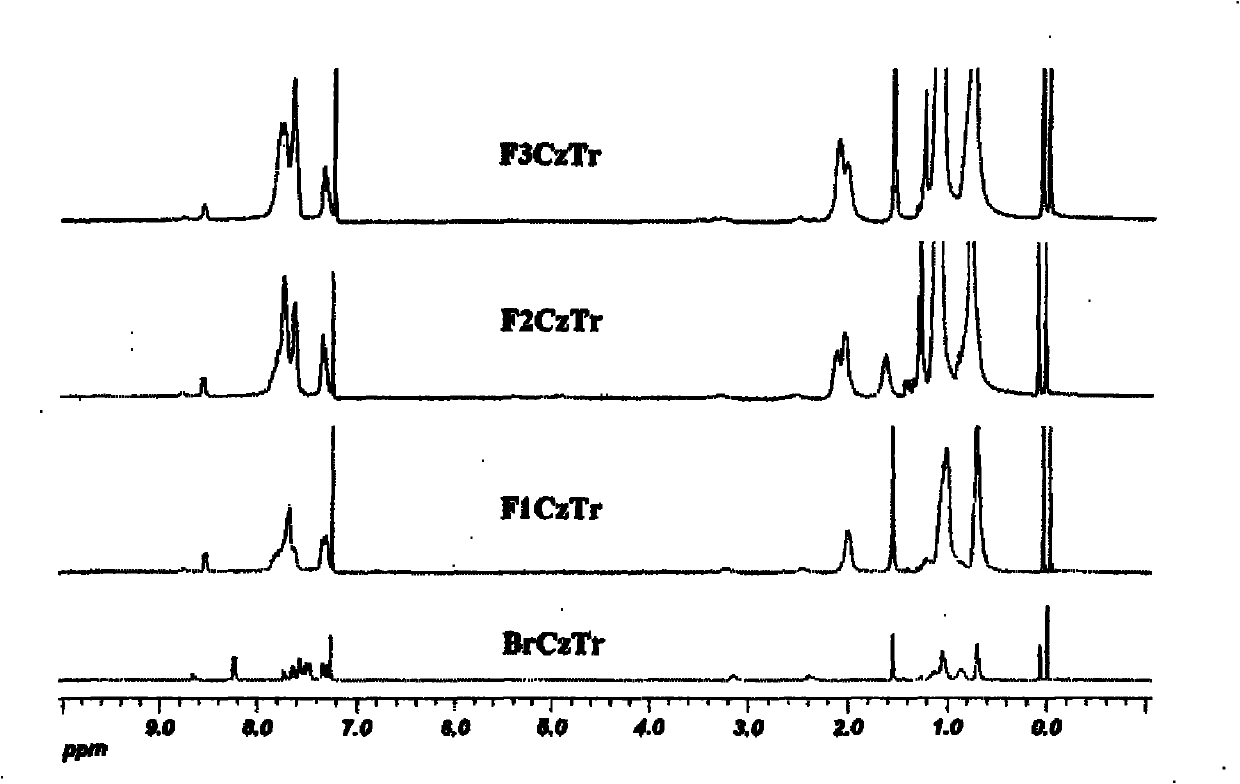
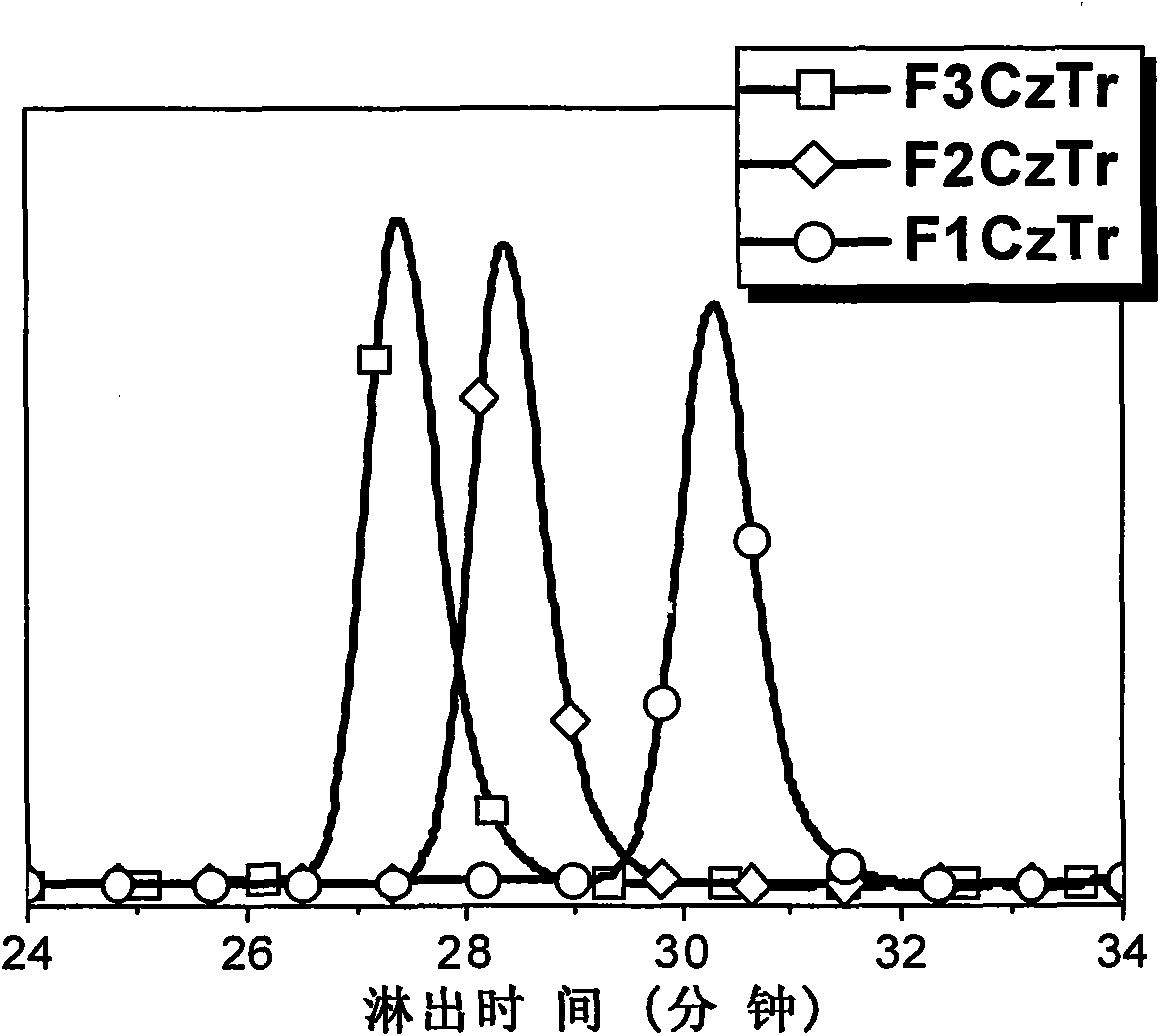
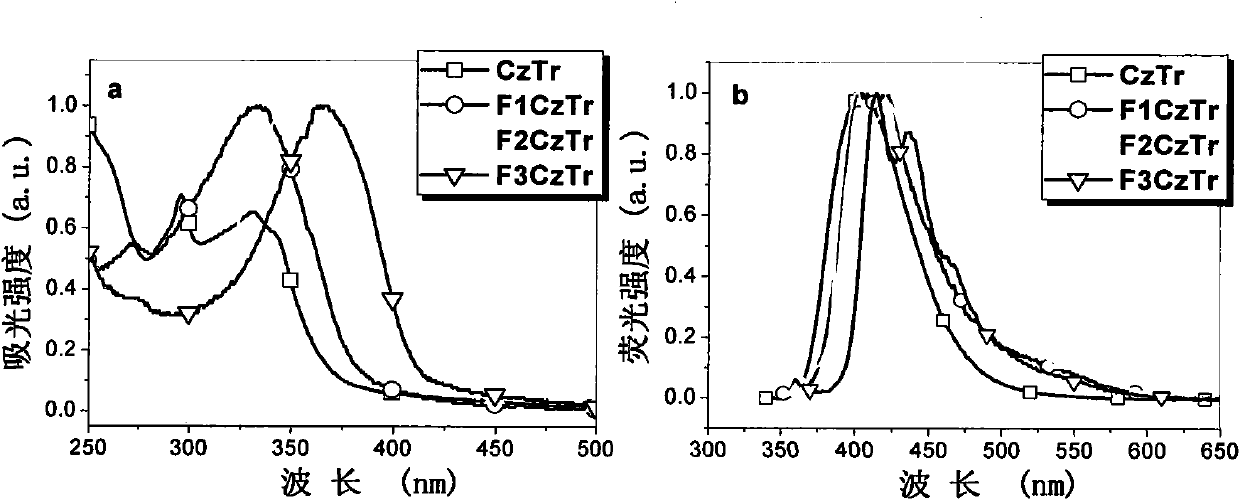
Multi-arm structure photoelectric-function material based on triindene elements
A photoelectric functional material, a technology of triindenes, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the change of luminous intensity and color purity, the chemical purity of polymer materials is not easy to improve, and restricts high-performance blue light polymerization In order to achieve good film-forming performance and good amorphous shape stability performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] target compound one
[0030] According to the reaction scheme 1, compound 1 and 2 were prepared by Suzuki coupling reaction under the condition of tetrakistriphenylphosphopalladium as catalyst and tetrahydrofuran as solvent to obtain compound CzTr. CzTr and NBS in CHCl 3 / DMF mixed solvent at 0°C to obtain the polybrominated intermediate BrCzTr. The product BrCzTr was subjected to multiple Suzuki coupling reactions with a unit of alkylfluorene boronate to obtain the target product F 1 CzTr.
[0031] 【Reaction Route 1】
[0032]
Embodiment 2
[0034]
[0035] target compound 2
[0036] According to the reaction scheme 2, the dialkylfluorene boronate is obtained from monobrominated dialkylfluorene through boration and esterification. Monobrominated dialkylfluorene is made of alkylfluorene borate and dibromoalkylfluorene under the catalysis of tetrakistriphenylphosphorous palladium, with 1,4 dioxane and two moles of potassium carbonate solution per liter As a solvent, it is prepared by reacting at 95°C. Multiple Suzuki Coupling Reaction of BrCzTr with Dialkylfluorene Boronate to Prepare the Target Product F 2 CzTr.
[0037] 【Reaction Route 2】
[0038]
Embodiment 3
[0040]
[0041] target compound three
[0042] According to reaction scheme 3, dibromoalkylfluorene borate and dibromoalkylfluorene are coupled by Suzuki to obtain monobrominated trialkylfluorene, and then borated and esterified to obtain triple alkylfluorene borate. Multiple Suzuki Coupling Reaction of BrCzTr with Triple Alkylfluorene Boronate to Prepare the Target Product F 3 CzTr.
[0043] 【Reaction route 3】
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com