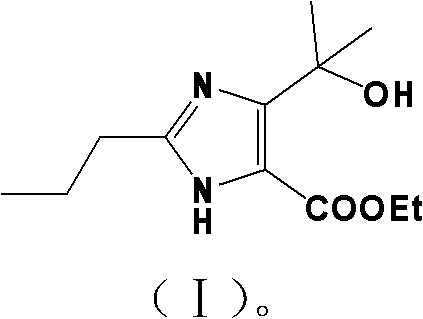
Method for synthesizing 4-(1-hydroxyl-1-methylethyl)-2-propyl iminazole-5-carboxylic ethyl ester
A technology of propylimidazole and methyl ethyl, which is applied in the field of chemical synthesis, can solve problems such as difficult precipitation of solids, influence on yield, crystal color, and poor decolorization effect by adding activated carbon, and achieve the effect of simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
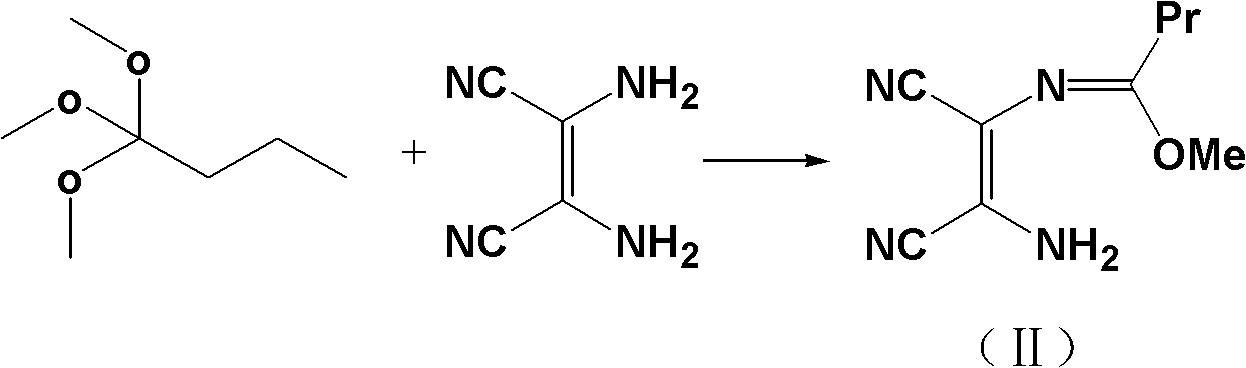
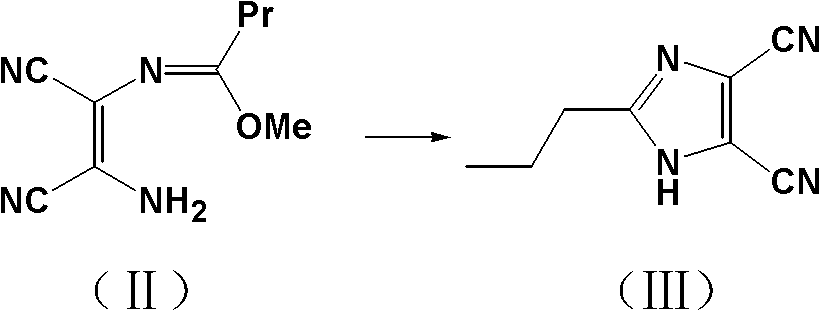
[0033] Example 1: Synthesis of 2-propylimidazole-4,5-dicarbonitrile (III).
[0034] In a 1500ml three-necked flask, add 200.0g (1.85mol) of diaminomaleonitrile and 300.0g (2.02mol) of trimethyl orthobutyrate and be dissolved in 500ml of anhydrous methanol, stir and reflux for 8 hours, and TLC detects that the reaction ends. Activated carbon (5g) was added to the system, the reaction was continued for 1 hour, filtered while it was hot, the filter cake was washed with 20ml of methanol, and ammonia gas (200g in total) was slowly introduced into the obtained filtrate in 3 batches under an ice bath, and the temperature of the system was maintained at 0-15°C. About 8 hours, TLC detects that the reaction is complete, 2 / 3 of methanol is recovered under reduced pressure, the residue is left standing to obtain block crystals, the crystals are filtered out, washed with cold methanol (50ml), and air-dried to obtain a white solid product with a yield of 99%. Content 99.8%, mp: 141~142.5℃, ...
Embodiment 2
[0037] Example 2: Synthesis of 2-propylimidazole-4,5-dicarbonitrile (III).
[0038] In a 1500ml three-necked flask, add 200.0g (1.85mol) of diaminomaleonitrile and 280.0g (1.90mol) of trimethyl orthobutyrate and be dissolved in 500ml of dehydrated alcohol, stir and reflux for 8 hours, TLC detection reaction ends, to this Activated carbon (5g) was added to the system, the reaction was continued for 1 hour, filtered while it was hot, the filter cake was washed with 20ml of ethanol, and ammonia gas (200g in total) was slowly introduced into the filtrate in 3 batches under an ice bath to maintain the temperature of the system at 0-15°C. About 8 hours, TLC detected that the reaction was complete, 2 / 3 of the methanol was recovered under reduced pressure, and the residue was allowed to stand still to obtain massive crystals, which were filtered off, washed with cold methanol (50ml), and air-dried to obtain a white solid product with a yield of 100%.
Embodiment 3
[0039] Example 3: Synthesis of diethyl 2-propylimidazole-4,5-dicarboxylate (IV).
[0040] Add 50.0 g (0.3 mol) of 2-propylimidazole-4,5-dinitrile (III) into a 1500 ml three-neck flask, slowly add 500 g of 20% (w / w) ethanol solution of hydrogen chloride dropwise, and reflux for 6 days , TLC showed that the reaction was complete, evaporated to dryness under reduced pressure, added saturated sodium bicarbonate solution (300ml) and ethyl acetate (100ml) to the residue and stirred at room temperature for 5 minutes, separated into layers, and the aqueous layer was washed with ethyl acetate (100ml×3) Extracted, combined organic layers, dried over anhydrous magnesium sulfate, filtered, and recovered ethyl acetate under reduced pressure to obtain white solid product 2-propylimidazole-4,5-dicarboxylated diethyl ester (IV), with a yield of 90%. The content is 99.7%. mp: 82~83.5℃, the detection parameters are as follows:
[0041] 1 H NMR (300MHz, CDCl 3 +D 2 O) δppm: 0.83 ~ 0.91 (3H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com