Nelarabine N-9 site alpha isomer, and preparation method and application thereof
A technology of N-9, isomers, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
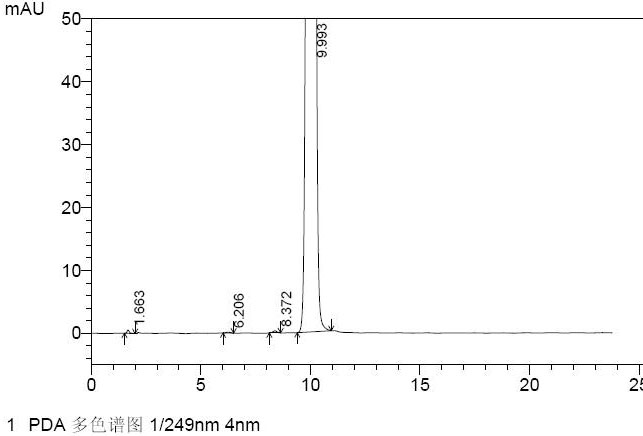
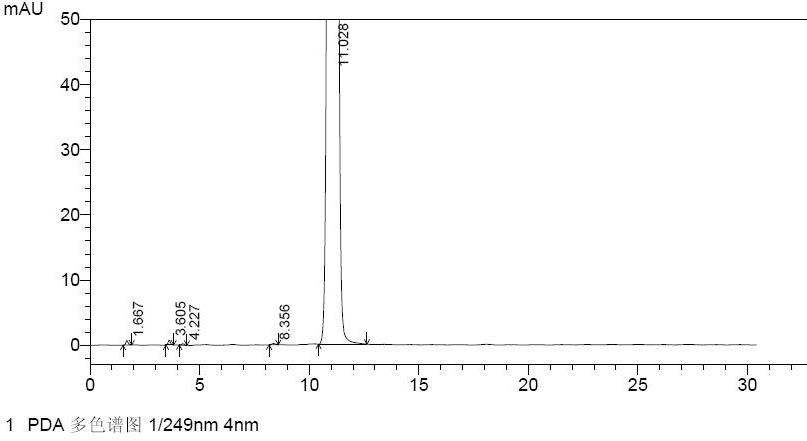
[0049] Example 1: Quantitative analysis of the N-9 alpha isomer in nelarabine
[0050] A. HPLC chromatography
[0051] Chromatographic column: Agilent Zorbax Bonus-Rp 4.6×150mm;
[0052] Mobile phase: 0.01mol / L disodium hydrogen phosphate solution (containing 0.6% triethylamine, adjust the pH value to 7.0 with phosphoric acid)-acetonitrile (95:5);
[0053] Detection wavelength: 249nm;
[0054] Flow rate: 1.0mL / min;
[0055] Column temperature: 40°C
[0056] Injection volume: 20μl
[0057] B. Prepare the standard solution positioning map for identifying the N-9 alpha isomer of nelarabine:
[0058] Prepare the solution of nelarabine N-9 α-isomer standard in the mobile phase, identify the N-9 α-isomer peak at the relative retention time RRT of 0.9, see the chromatogram Figure 1 .
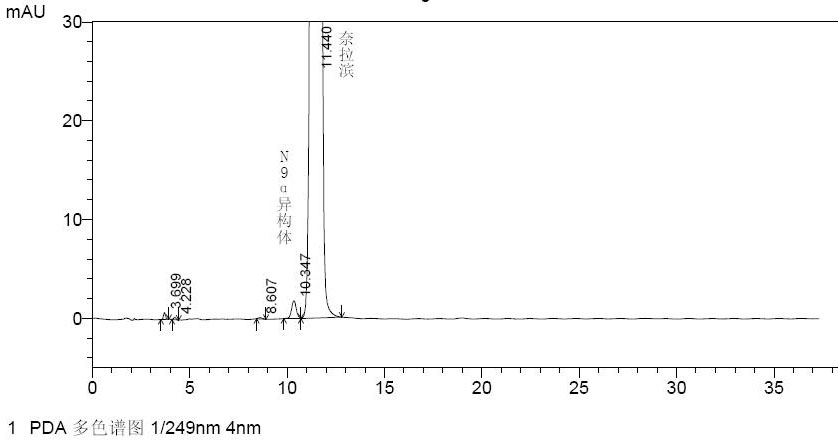
[0059] C. Preparation of a marker solution for identifying the N-9 alpha isomer of nelarabine:
[0060] Prepare a nelarabine marker solution containing the N-9 alpha isomer of nelarabine at a ...
Embodiment 2
[0066] Example 2 Preparation of 2-amino-6-chloro-9-D-(2,3,5-tri-O-benzyl-arabinofuryl)-9H-purine (intermediate A)
[0067] Add 152.6 g (0.9 mol) of 2-amino-6-chloropurine and 2300 g of hexamethyldisilazane into the reaction flask, stir for 10 minutes, then add 147 g (1.35 mol) of trimethylchlorosilane, heat to 120°C, The reaction was kept for 9 hours, the solid basically dissolved to form a solution, and concentrated under reduced pressure to dryness to obtain the silylated product of 2-amino-6-chloropurine, which was directly used in the next reaction.
[0068] The 2-amino-6-chloropurine silylated product obtained in the previous step was placed in a 20L reaction flask, and then 355g (0.81 mol) and 9,000 g of 1,2-dichloroethane, add 200 g (0.9 mol) of trimethylsilyl trifluoromethanesulfonate (TMSOTf) dropwise at 20-30°C under controlled internal temperature, and React for 12 hours, after TLC monitors that the raw material point disappears, pour the reaction solution into 500...
Embodiment 3
[0069] Example 3 Preparation of 2-amino-6-methoxy-9-α-D-(2,3,5-tri-O-benzyl-arabinofuranosyl)-9H-purine (intermediate B)
[0070] Put 11.4g of intermediate A (0.02mol) prepared according to Example 2 into a reaction flask, add 114mL of methanol, under magnetic stirring, add 3.6g (0.067mol) of sodium methoxide, heat up to 60°C and keep it warm for 30 minutes, The reaction at the raw material point was detected by TLC, and the reaction solution was concentrated and evaporated to dryness to obtain an oily substance; 200mL of dichloromethane and 200mL of purified water were added to dissolve, and the water layer was separated, and the water layer was extracted twice with 100mL of dichloromethane, and the organic layer was combined, saturated with chlorine Sodium chloride solution 100mL × 2 washes, the organic layer was dried with anhydrous sodium sulfate, filtered, concentrated and evaporated to dryness to obtain a light yellow oil;
[0071] Dissolve the sample with dichloromethan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com