Method for realizing over expression of thermostable laccase gene through location transformation
A technology of overexpression and heat resistance, applied in the fields of genetic engineering, enzymology, and molecular biology, can solve the problems of low yield and poor stability, and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (2) Using the Thermus thermophilus genomic DNA as a template, PCR amplification of the laccase gene was carried out with the above primers.
[0042] (3) with Pst I and Sph I process the PCR product and compare it with the Pst I and Sph I double-digested heat shock expression vector pHsh ligation.
[0043] (4) Imported by electroporation E. coli After DH10B, apply to LB plates containing 0.1 mg / ml ampicillin and culture at 30°C.
[0044] (5) Pick a single colony from the LB plate, inoculate them into 4 ml LB liquid medium containing 0.1 mg / ml ampicillin, culture at 30°C overnight, and extract the plasmid according to the standard method.
[0045] (6) The plasmid was sent to Huada Gene Technology Co., Ltd. for sequence determination. The plasmid with the same DNA sequence as TTC1370 was named pHsh- lcs -0, and used as starting material and experimental control for subsequent studies.
[0046] Example 2: Site-directed mutagenesis of the extremely thermostable ...
Embodiment 2
[0048] (2) Design and synthesize a pair of reverse long primers to replace the rare and non-dominant codons in the 1st to 27th codons. The sequence of the primers is: 5'-ACGAACAACT TTCGGTTCCG GAAAGCTCGG ACCCTGCATGG GTATATCTCC TTCTTG-3' (SEQ ID NO: 8), 5'-AGCCAGGGTG GTCTGCTGAG CCTGAAACTG AGCGCAACCC CGACCCCGCT TGCCCTGG-3' (SEQ ID NO: 9).
[0049] (3) Take pHsh- lcs -0 is the template, and the above primers are used for PCR amplification. The PCR conditions were set as: 95°C, 5 min; pause the timer, add Pyrobest polymerase, add 40 mL of paraffin oil for sealing; 35 cycles (94°C, 30 s; 60°C, 60 s; 72°C, 4 min); 72 ℃, 10 min; stop the reaction, keep warm at 4℃.
[0050] (4) Separate the PCR products and templates by agarose gel electrophoresis, and recover the DNA fragments in the PCR amplified bands by tapping the gel. After the PCR product was treated with T4 DNA kinase to phosphorylate it, T4 DNA ligase was added to self-circularize it.
[0051] (5) Imported by electroporat...
Embodiment 3
[0055] Example 4: Next round of site-directed mutagenesis of the extremely thermostable laccase gene
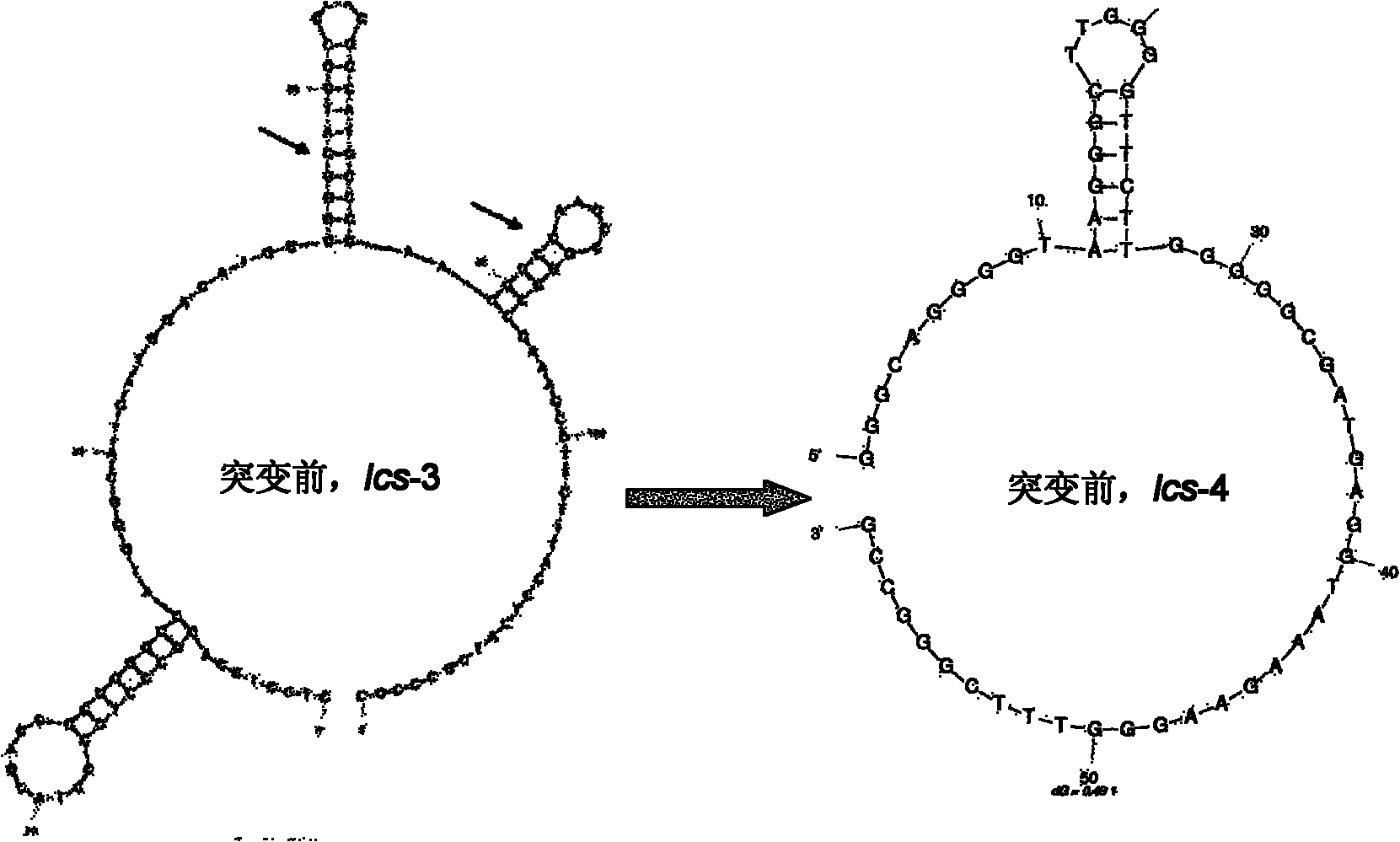
[0056] (1) In the experiment, the gene sequencing signal was often interrupted in a GC base-rich region (GC island) of the laccase gene, and the DNA online analysis software mfold (http: / / frontend.bioinfo.rpi.edu / applications / mfold) analysis found that GC islands lead to the formation of complex secondary structures in DNA, and the mutant designed in the present invention can reduce this structure ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com