Composite ceramic powder, process for production of same and solid oxide fuel cell
A composite ceramic and manufacturing method technology, applied in solid electrolyte fuel cells, cobalt compounds, nickel compounds, etc., can solve the problems of large primary particle size, uneven mixing, lack of sufficient characteristics, etc., to increase oxygen ions The effect of chemical quantity, many three-phase interfaces, and excellent composition controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
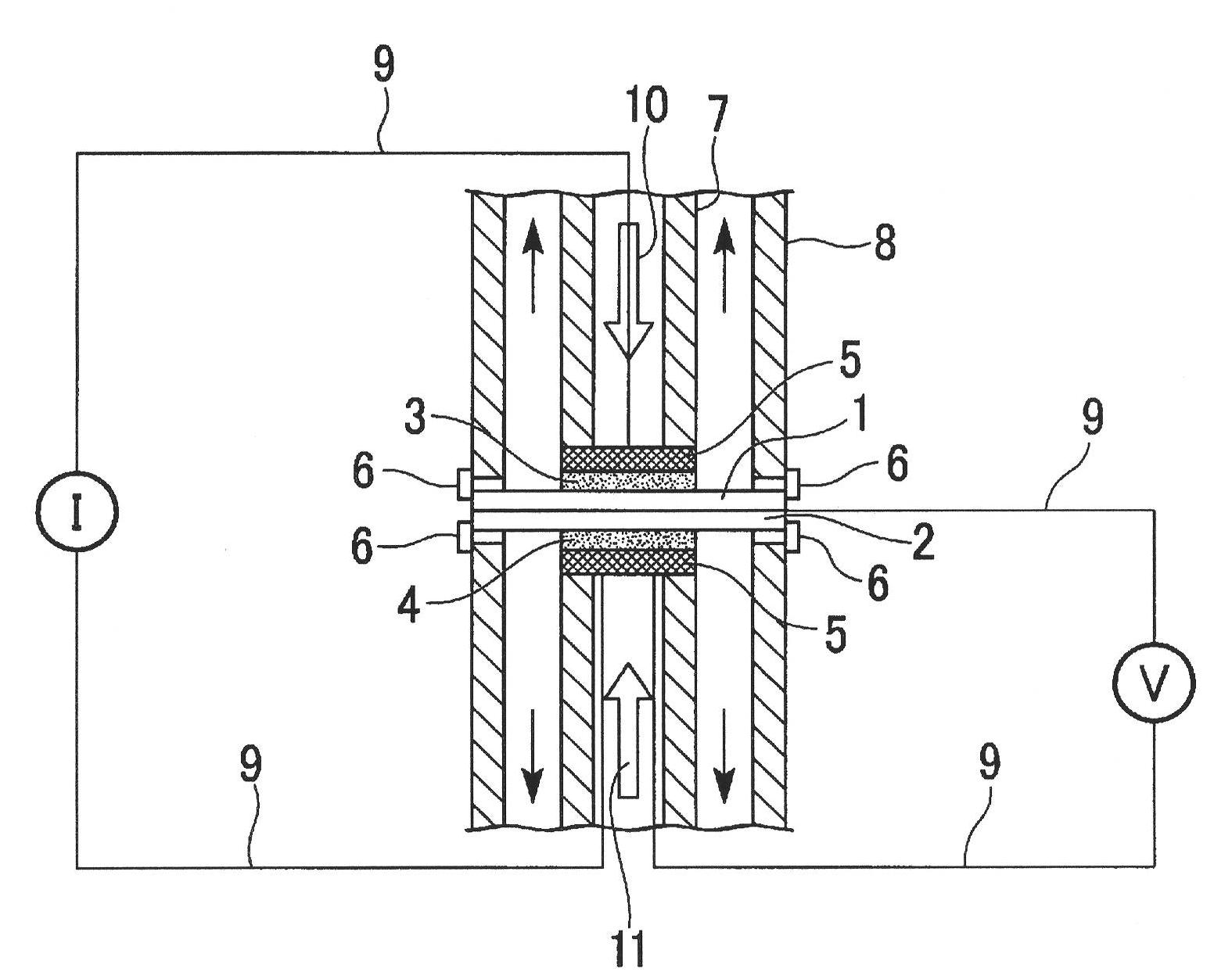
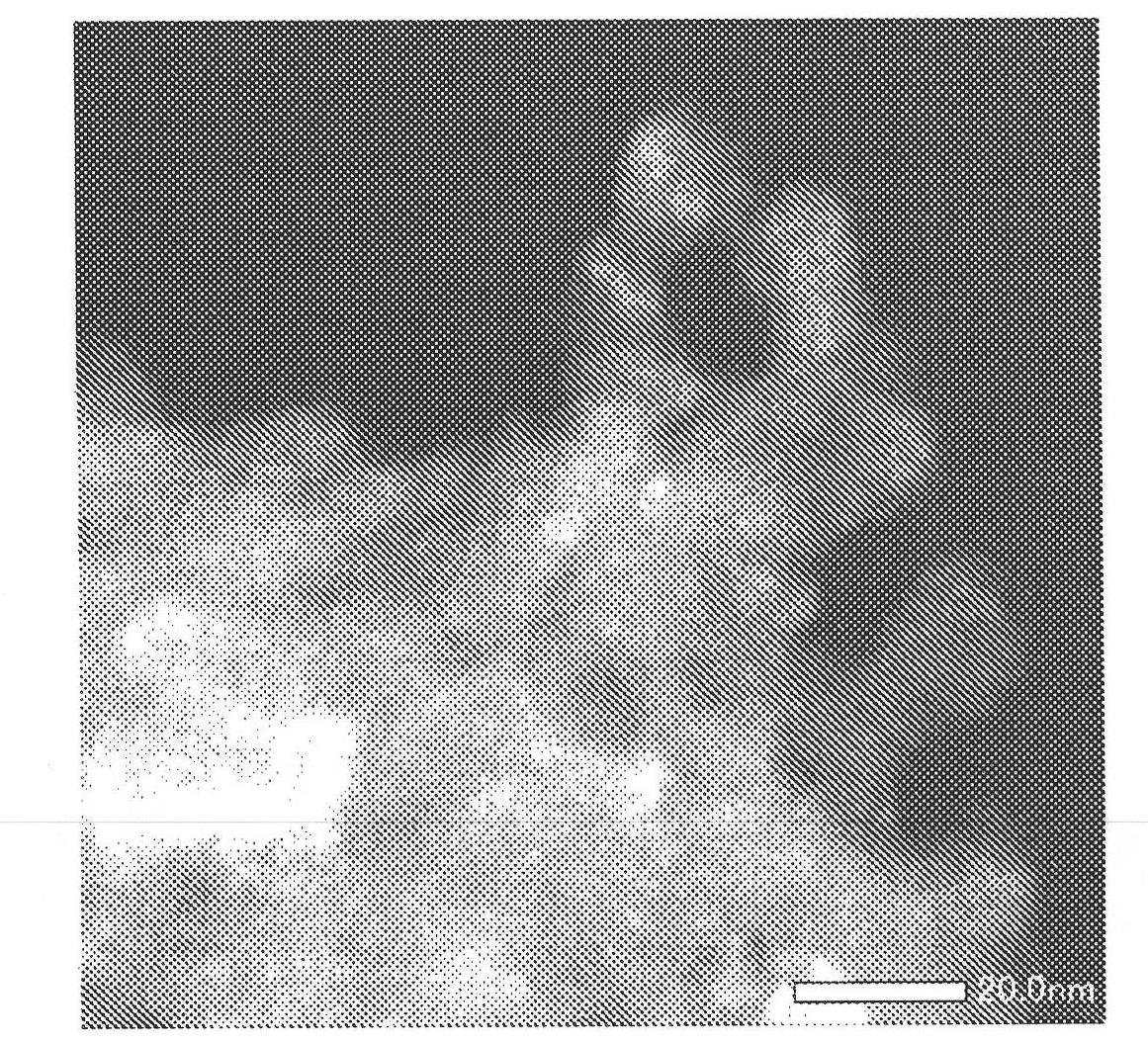
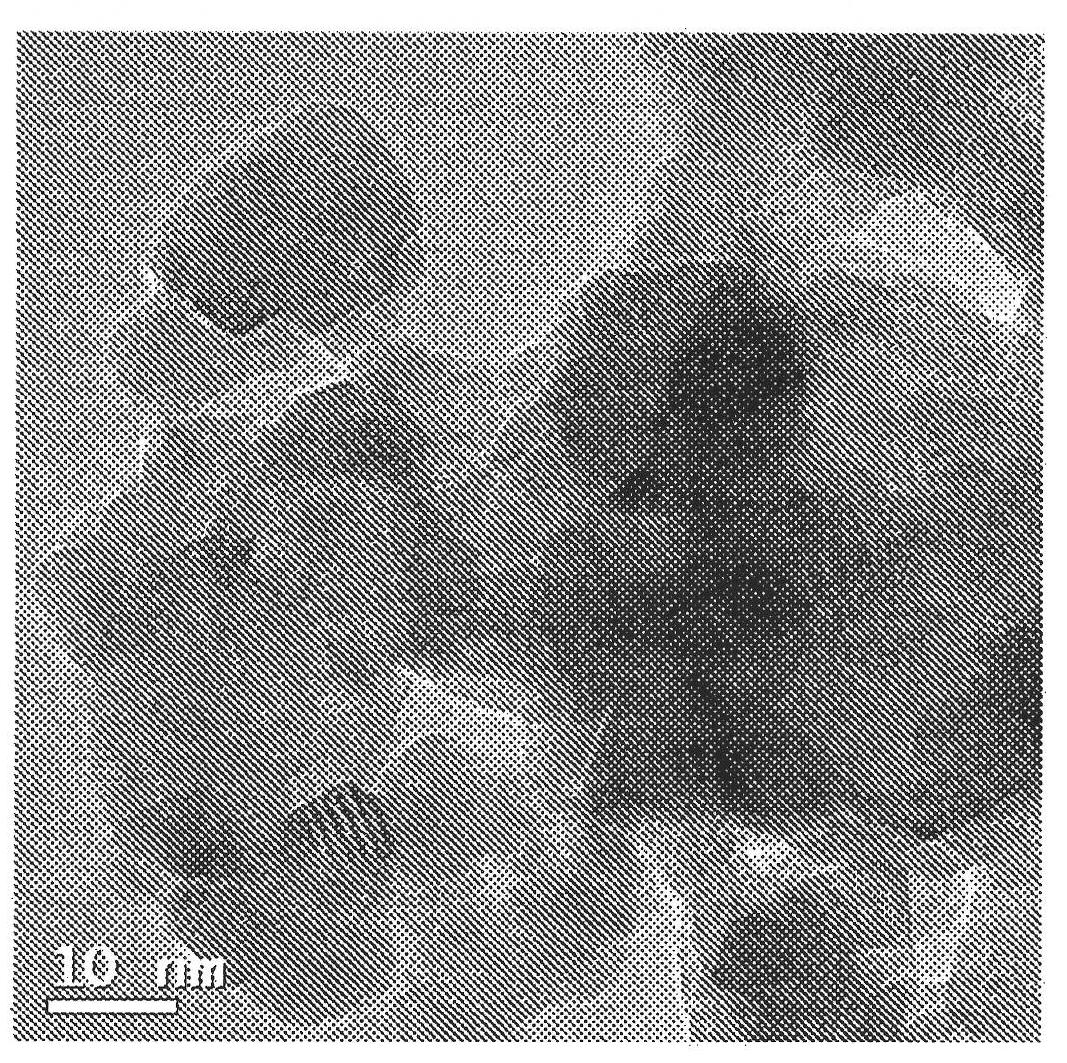
Image
Examples
Embodiment approach 1
[0056] [Composite ceramic powder]
[0057] The composite ceramic powder according to Embodiment 1 of the present invention is a powder containing 1-x B x C 1-y D. y o 3 (In the formula, A is one or two elements selected from the group of La and Sm, B is one or more elements selected from the group of Sr, Ca and Ba, C is selected from Co and Oxide and zirconia represented by one or two elements in the group of Mn, D is one or two elements selected from the group of Fe and Ni, and 0.1≤x≤0.5, 0≤y≤0.3) And constitute the composite ceramic powder; 1-x B x C 1-y D. y o 3 The zirconia acidic dispersion liquid of embodiment 1 in which one or more ions in the group of A, B, C and D are added to the alkaline solution to obtain the neutralized precipitate of embodiment 1, And the powder formed by heat-treating the neutralized precipitate.
[0058] It is preferable that the dispersed average particle size of the zirconia particles in the zirconia acidic dispersion liquid accord...
Embodiment approach 2
[0102] [Composite ceramic powder]
[0103] The composite ceramic powder according to Embodiment 2 of the present invention is a composite ceramic powder composed of nickel oxide and zirconia, and is a composite ceramic powder according to Embodiment 2 containing zirconia particles composed of yttria-stabilized zirconia and nickel ions. A zirconia acidic dispersion liquid is added to an alkali solution to obtain the neutralized precipitate of Embodiment 2, and the neutralized precipitate is heat-treated to form a powder.
[0104] It is preferable that the dispersed average particle size of the zirconia particles in the zirconia acidic dispersion liquid according to Embodiment 2 is 20 nm or less.
[0105] The composite ceramic powder according to Embodiment 2 is produced by adding the zirconia acidic dispersion liquid of Embodiment 2 containing zirconia particles made of yttria-stabilized zirconia and nickel ions to an alkaline solution to produce Embodiment 2. Then, the neutra...
Embodiment 1
[0130] Lanthanum nitrate [La(NO 3 ) 3 ·6H 2 O] 62.81g, strontium nitrate [Sr(NO 3 ) 2 〕7.68g, manganese nitrate [Mn(NO 3 ) 2 ·6H 2 O) 52.04g is dissolved in 1000g of dilute nitric acid with a pH of 2.0 to make La 0.8 Sr 0.2 MnO 3 The metal salt aqueous solution of La, Sr and Mn of the composition is stirred, and the 10mol% yttria-stabilized zirconia (LSM-10YSZ) acid dispersion (pH: 2.0) containing La, Sr and Mn ions is produced (dispersion A- 1).
[0131] Then, dissolve ammonium bicarbonate (NH 4 HCO 3 ) 75.72 g to prepare ammonium bicarbonate aqueous solution (alkaline carbonic acid aqueous solution) (aqueous solution B-1).
[0132]Then, the dispersion liquid A-1 was dropped into the aqueous solution B-1 to obtain a neutralized precipitate. Here, a 25% by mass ammonia solution was dropped into the aqueous solution B-1 simultaneously with the dispersion A-1 to keep the pH of the aqueous solution B-1 at 8.
[0133] Then, the obtained neutralized precipitate was wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com