Method for degrading methyl orange dye wastewater with ZnIn2S4 visible light catalyst
A dye wastewater, znin2s4 technology, applied in chemical instruments and methods, light water/sewage treatment, water pollutants, etc., can solve the problems of high cost, high energy consumption, and long time to degrade wastewater, and achieve short time and energy consumption Effects of small, mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
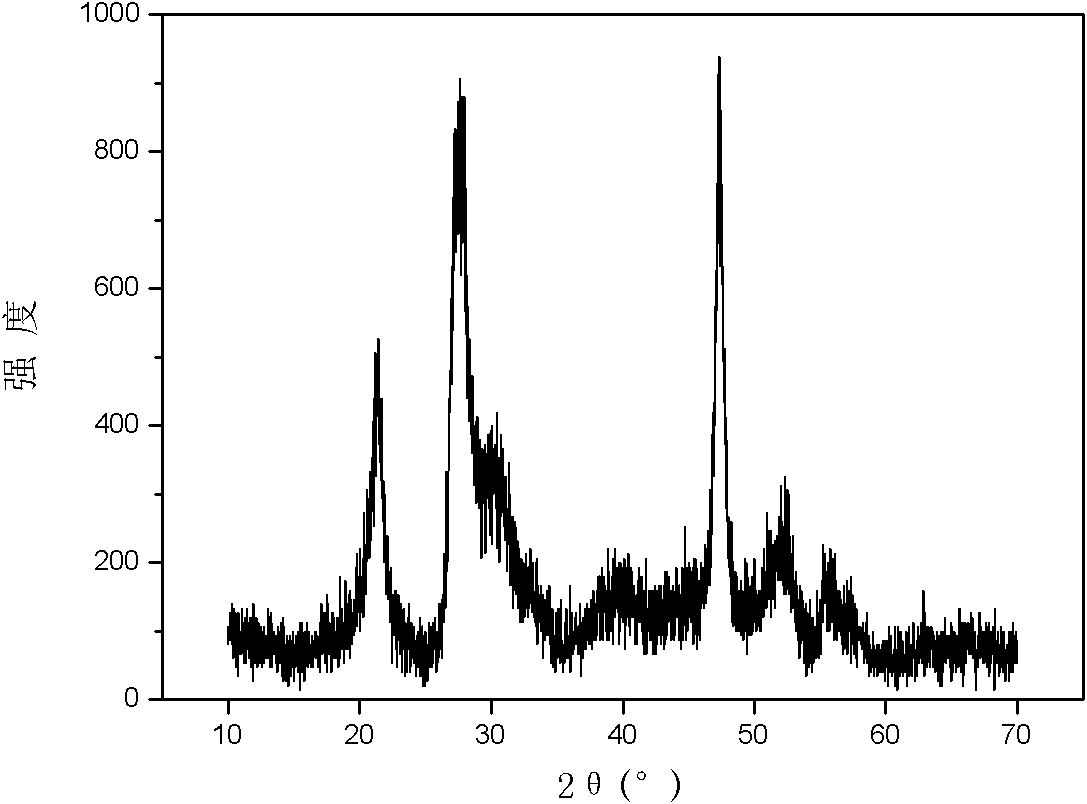
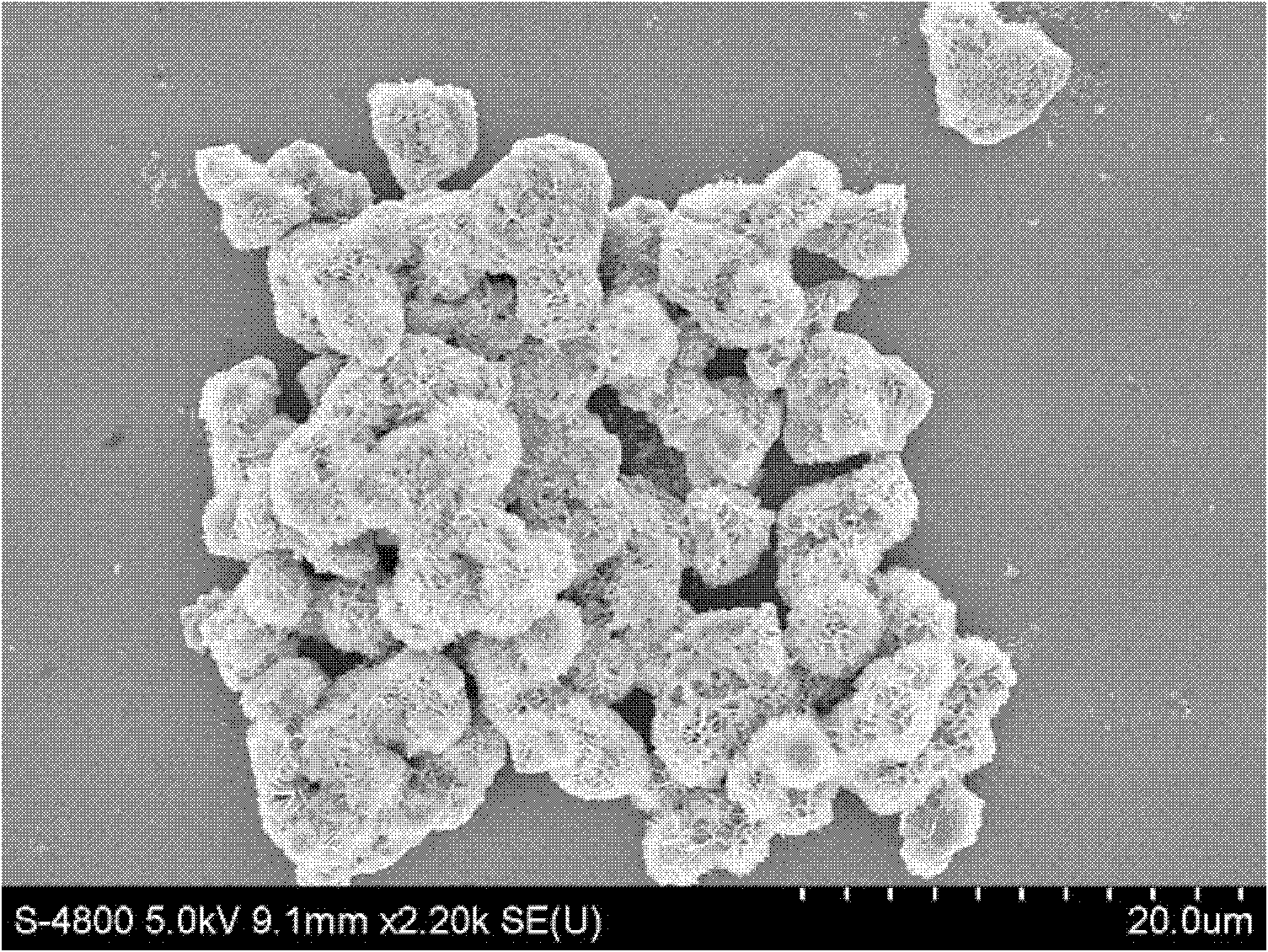
[0029] Accurately weigh 0.25mmol Zn(NO 3 ) 2 ·6H 2 O, 0.5mmol In(NO 3 ) 3 4.5H 2 0 and 1.5mmol of thioacetamide were put into a 50mL polytetrafluoroethylene liner, accurately pipette 15mL of deionized water as a solvent with a pipette gun, and obtained a colorless and clear solution after magnetic stirring for 40min, sealed a stainless steel reaction vessel, and React at 90°C for 16h. Cool, wash, and dry to obtain a yellow powder. The yellow powder was subjected to X-ray powder diffraction (attached figure 1 ) identified as hexagonal ZnIn 2 S 4 , and there are no impurities in the product. The morphology was characterized by scanning electron microscopy (attached figure 2 ) is microspherical or irregular, with a particle size of 20um-100um.
[0030] Accurately pipette 250mL methyl orange dye wastewater and place it in a glass reactor with a volume of 250mL. Add 0.1g ZnIn 2 S 4 Particle powder, the reaction system was placed in a dark room, and stirred for 1 h i...
Embodiment 2
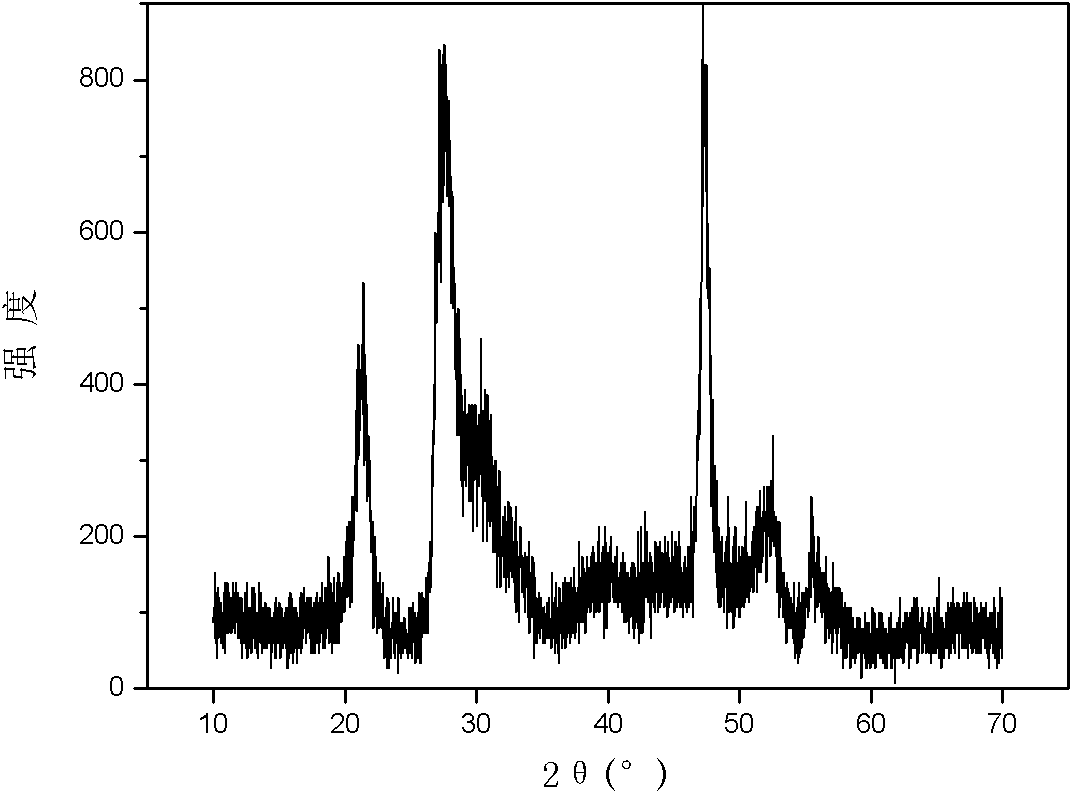
[0032] Repeat Example 1, change the synthetic temperature to be 70 DEG C, other conditions are constant, the product obtained is through X-ray powder diffraction (attached image 3 ) identified as hexagonal ZnIn 2 S 4 . The reaction results are: after 1 hour of light, the degradation rate of methyl orange is 43.1%; after 2 hours of light, the degradation rate is 79.8%; after 2.5 hours of light, the degradation rate is 93.5%; after 3 hours of light, it is completely degraded.
Embodiment 3
[0034] Repeat Example 1, change synthesis temperature to be 55 ℃, other conditions are constant, gained product is through X-ray powder diffraction (attached Figure 4 ) identified as hexagonal ZnIn 2 S 4 , the morphology was examined by scanning electron microscope (attached Figure 5 ) are characterized as being microspherical or irregular. The reaction results are: after 0.5h of light, the degradation rate of methyl orange is 51.3%; after 1h of light, the degradation rate is 83.5%; after 1.5h of light, it is completely degraded.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com