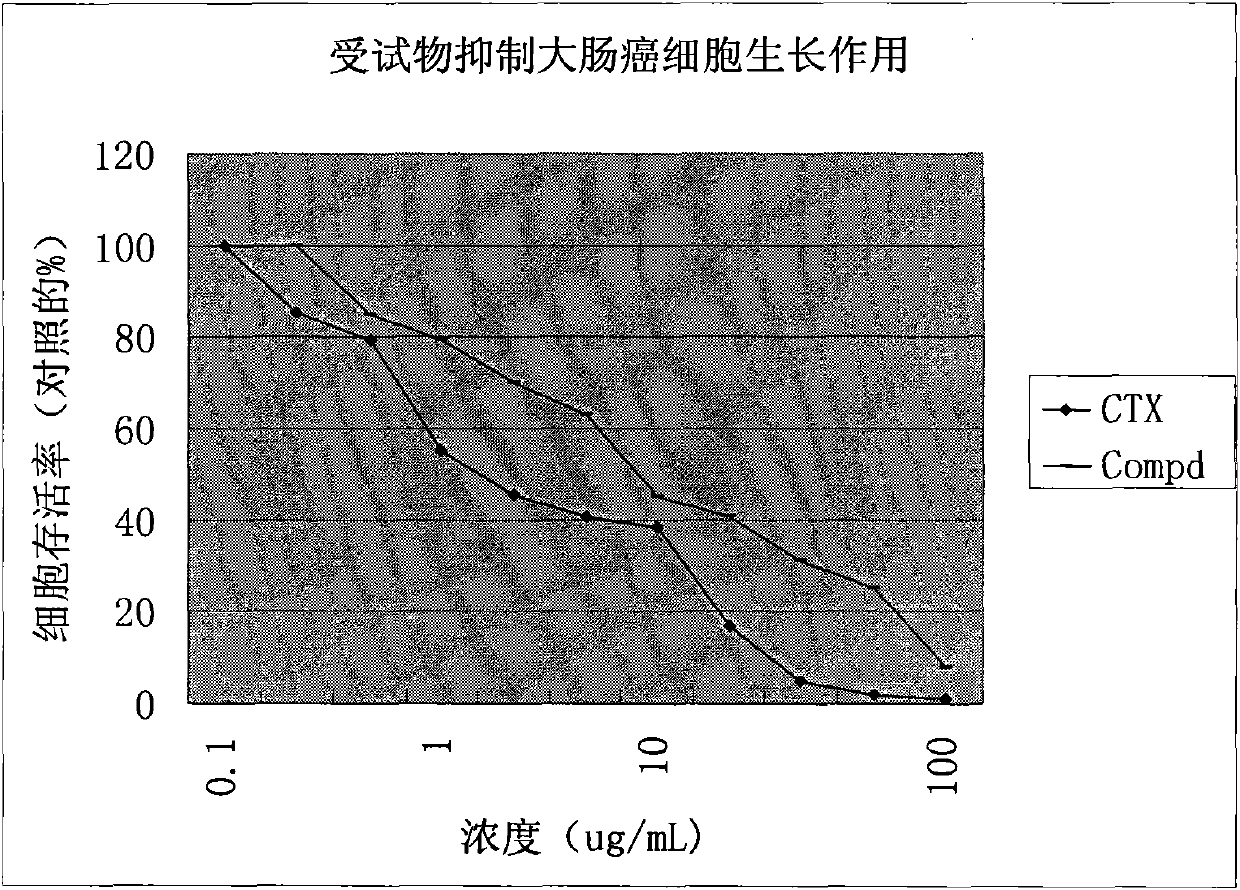
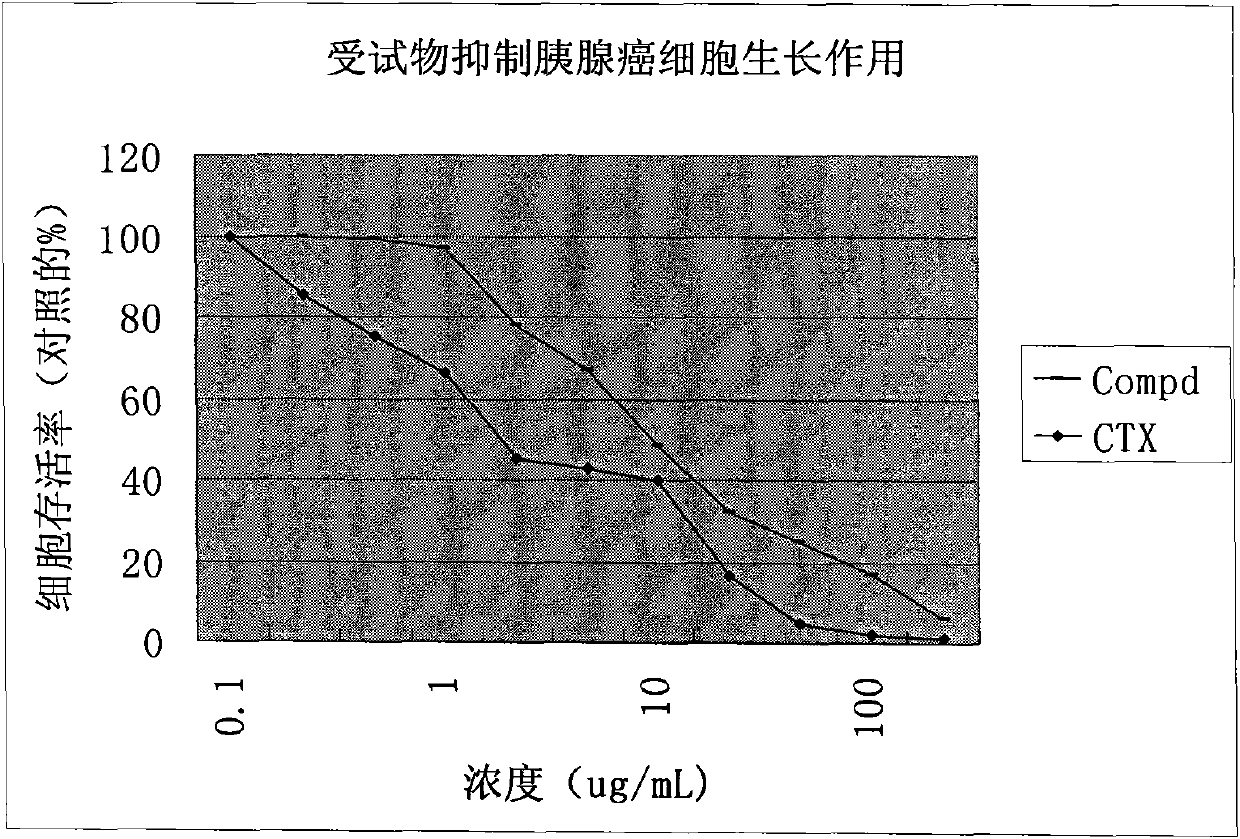
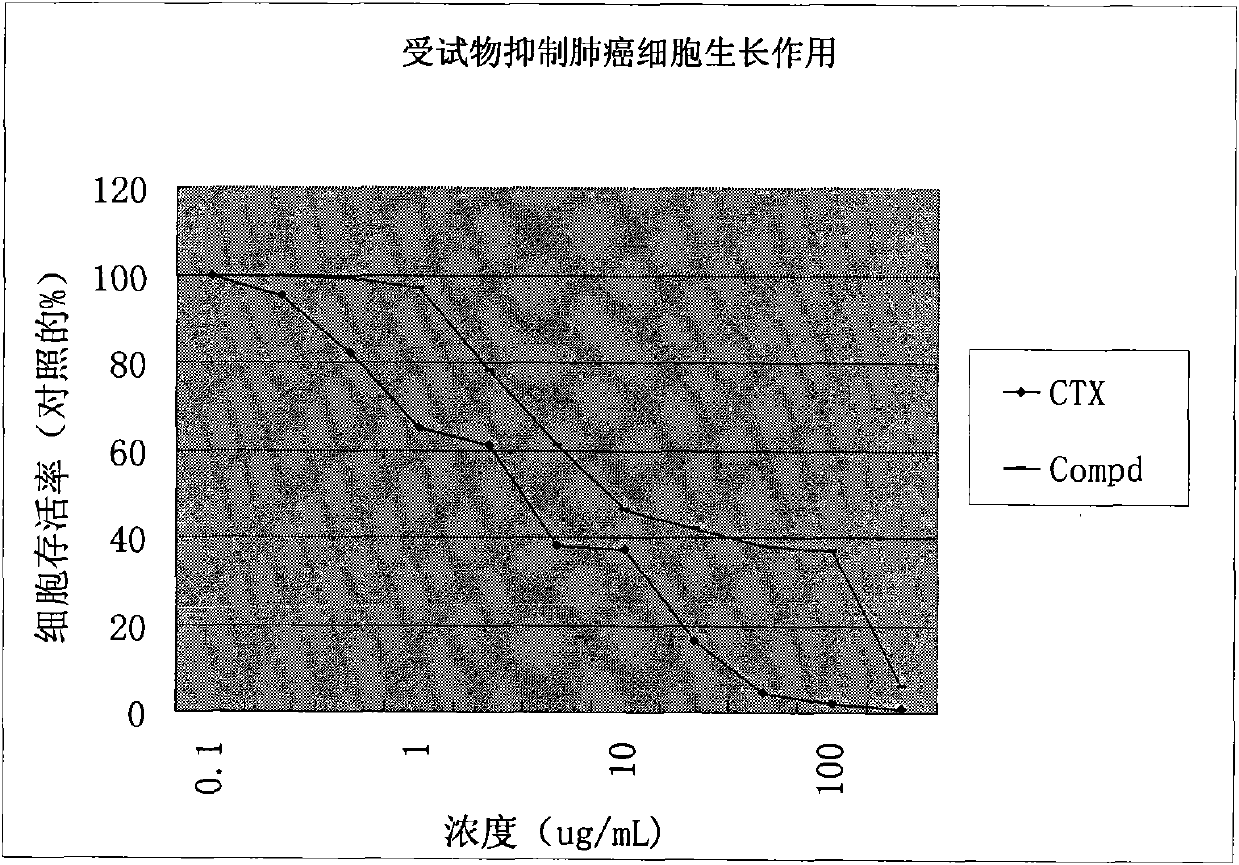
Medicine tromantadine with adamantane structure, anti-tumor application of derivates and analogs thereof having new indications
A kind of technology of tramandamide and derivatives, applied in the application field of disease medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] 3. Preparation of salts of test compounds
[0025] Take a certain amount of this product, and dissolve it quantitatively in methanol, acetone, 4-hydrofuran or 1,4-dioxane. If the dissolution is not good, you can properly heat it on the basis of keeping the compound stable to make it a true solution. Cool in an ice-water bath and stir at this temperature to slowly feed dried hydrogen chloride gas until it is completely saturated, and then keep feeding it for 5 minutes. Keep the salt-forming reaction for 1-2 hours, and detect the reaction by thin-layer chromatography until all the salts are formed. Evaporate the solvent under reduced pressure and dry under reduced pressure.
[0026] Pharmaceutically acceptable salts of the compounds of the present invention are also within the scope of the present invention, and their acids can be salted by reacting with bases, such as sodium carbonate, sodium hydride, potassium hydroxide, ammonium hydroxide and the like. Containing a n...
Embodiment 1
[0032] Embodiment 1. Structural identification of tromantine
[0033] 1 H NMR (CDCl 3 )δ6.78(s, 1H), 3.83(s, 2H), 3.75(m, 2H), 2.51(m, 2H), 2.28(s, 6H), 2.07(s, 3H), 2.02(m, 6H ), 1.68 (m, 6H); MS / e: 281 (M+1).
Embodiment 2
[0035] Anti-tumor preparation: Weigh 8.0g tromantine, add 50ml DMSO, stir to dissolve, add 500ml 1,2-propanediol and 100ml Tween 80 after dissolving, stir and mix evenly, add water for injection to a total volume of 5000ml, use 0.22μm Membrane filtration, packaging, autoclaving at 100°C for 30 minutes, leak detection, full inspection, packaging, that is, 8mg / 5ml (ammonia bottle), a total of 1000 pieces.
[0036] In vitro anti-tumor experiment example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com