Method for purifying 3,4,5,6-tetrachloro-2-cyanopyridine by sublimation and catching, and catcher and system thereof
A technology of cyanopyridine and traps, which is applied in the field of desublimation traps and device systems, can solve the problems of difficult automatic control, complex control of process conditions, and no mention of trapping, so as to improve the quality of by-product hydrochloric acid , Easy operation and control, energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
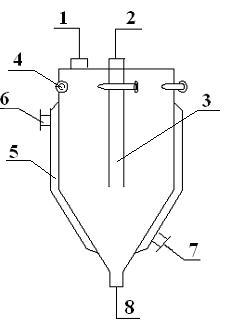
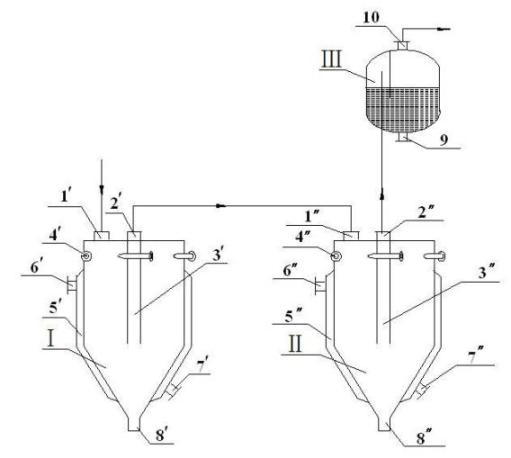
[0036] according to image 3 The device system for desublimation trapping and purification of 3,4,5,6-tetrachloro-2-cyanopyridine shown in the present invention, → indicates the direction of the material gas, and the device system includes the first trap I, The second trap II and bubble absorber III, the first trap I and the second trap II have the same structure (such as figure 1 and figure 2 shown), wherein the gas outlet 2' of the first trap is connected to the material gas inlet 1" of the second trap, and the gas outlet 2" of the second trap is communicated with the bubble absorber; The top of the bubbling absorber is provided with an exhaust outlet 9, and the bottom of the bubbling absorber is provided with a discharge port 10.
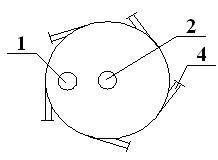
[0037] like figure 1 and figure 2 As described above, the trap of the present invention is a closed cavity formed by combining an upper cylinder and a lower cone, wherein a material gas inlet 1 is provided on the top side of the cylinder, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com