Preparation method of orlistat
A technology of orlistat and preparation column, applied in the direction of organic chemistry, etc., can solve problems such as the simplicity of operation steps, amplification linearity and yield, and no significant breakthrough in purification process, so as to achieve easy production control, reduce Effects of solvent consumption and shortened production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
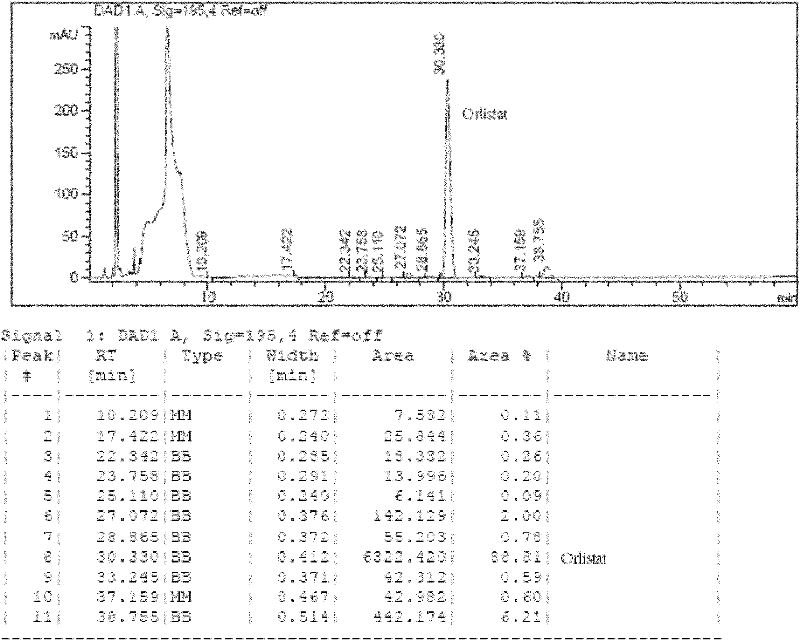
Image
Examples
Embodiment 1
[0042] Mix about 80Kg with a particle size of 10μm and a pore size of Pour the reversed-phase C18 spherical silica gel filler into about 150L of isopropanol, stir evenly to make a filler slurry, remove filler fragments and fine powder, quickly pour it into a DAC preparation column with an inner diameter of φ800mm, and set the column packing pressure to 20MPa , Open the pneumatic piston rod of the column packing machine for axial compression, and at the same time open the valve at the lower end of the DAC preparation column to discharge and recover the isopropanol. When the piston is compressed to the set pressure of 20MPa, the column packing is completed, the height of the column bed is 250mm, and the specification of the column bed is φ800mm×250mm. The installed DAC preparative column has been tested for column efficiency, and the number of theoretical plates reaches 46812N / m.
[0043] Liprestatin (purity 87.46%, content 66.20%) was dissolved in 86% methanol solution, and t...
Embodiment 2
[0049] Mix about 75Kg with a particle size of 10μm and a pore size of Pour the reversed-phase C8 spherical silica gel filler into about 150L of isopropanol, stir evenly to make a filler slurry, remove filler fragments and fine powder, and quickly pour it into a DAC preparation column with an inner diameter of φ800mm, and set the column packing pressure to 24MPa , Open the pneumatic piston rod of the column packing machine for axial compression, and at the same time open the valve at the lower end of the DAC preparation column to discharge and recover the isopropanol. When the piston is compressed to the set pressure of 24MPa, the column packing is completed, the height of the column bed is 250mm, and the specification of the column bed is φ800mm×250mm. The installed DAC preparative column has been tested for column efficiency, and the number of theoretical plates reaches 42663N / m.
[0050] Liplastatin (purity 87.46%, content 66.20%) was dissolved in 85% methanol solution, th...
Embodiment 3
[0055] Mix about 10Kg with a particle size of 15 μm and a pore size of Pour high-pressure resistant reverse-phase polymer resin filler based on polystyrene / divinylbenzene into about 20L of isopropanol, stir evenly to make filler slurry, remove filler fragments and fine powder, and pour it quickly to an inner diameter of φ300mm In the DAC preparation column cylinder, set the pressure to 10MPa, open the pneumatic piston rod of the column packing machine to perform axial compression, and simultaneously open the valve at the lower end of the DAC preparation column to discharge and recover the isopropanol. When the piston is compressed to the set pressure of 10MPa, the column packing is completed, the height of the column bed is 300mm, and the specification of the column bed is φ300mm×300mm. The installed DAC preparative column has been tested for column efficiency, and the number of theoretical plates reaches 38764N / m.
[0056] Liprestatin (purity: 89.17%, content: 64.56%) was d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com