Polymer for fluorescein angiogram and isotope angiogram
A polymer and isotope technology, applied in preparations for in vivo experiments, luminescent materials, chemical instruments and methods, etc., to achieve the effects of reduced interference, high resistance to photobleaching, and stable fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
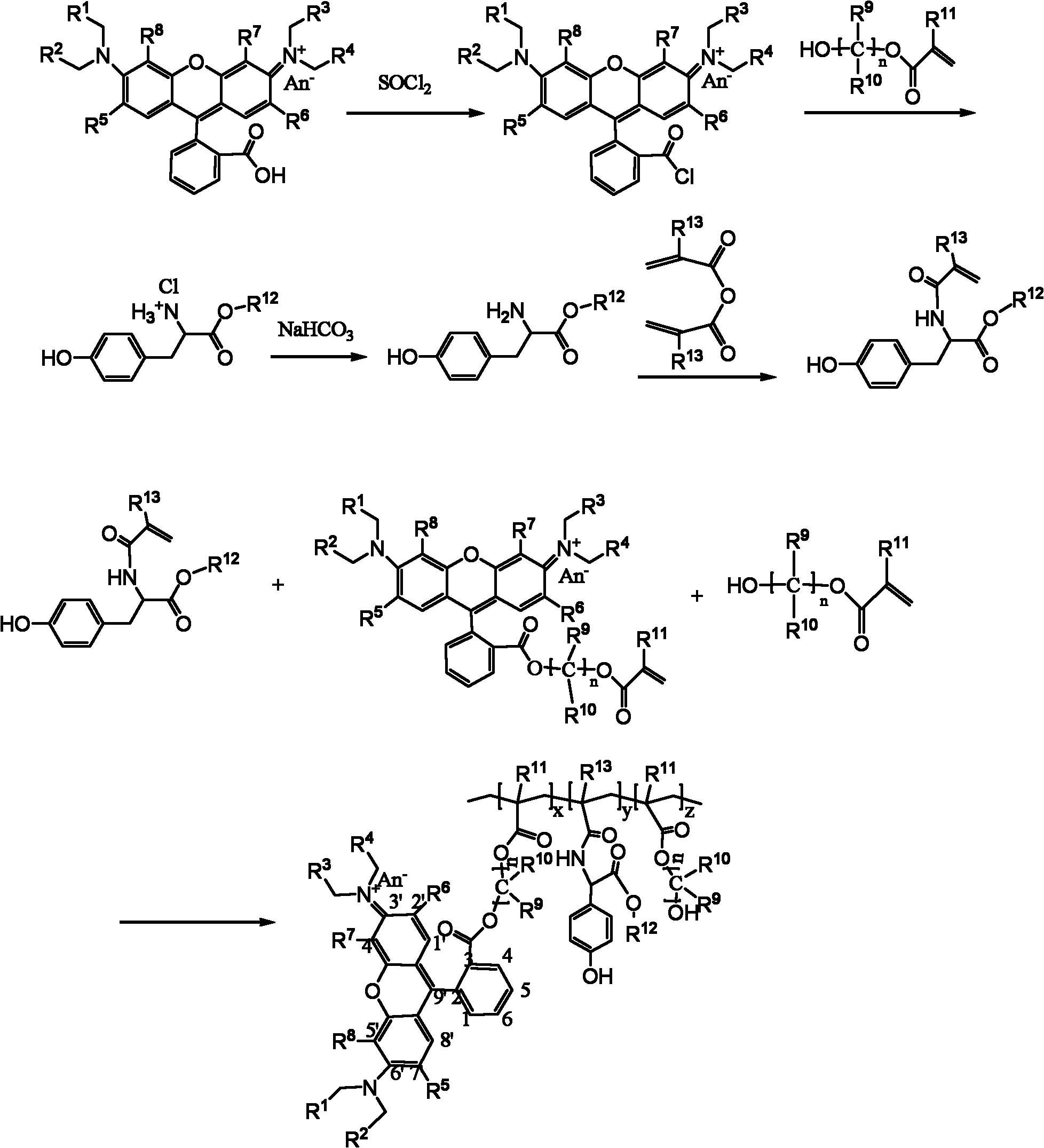
[0033] Embodiment one: with reference to attached figure 1 To synthesize polymers that can be used for fluorescence contrast and isotope contrast, the specific steps are as follows:
[0034] Synthesis of monomers containing rhodamine B groups:
[0035] 1 g of rhodamine B (2.1 mmol) was dissolved in 1,2-dichloroethane, and 0.9 ml of thionyl chloride was slowly added dropwise at room temperature. The reaction system was stirred and refluxed for 6 hours. After the reaction was completed, the solvent was removed under reduced pressure to obtain the crude product of rhodamine B acid chloride. Add dichloromethane and stir to dissolve Rhodamine B chloride completely. 1.0 ml (9.6 mmol) of hydroxyethyl acrylate was slowly added dropwise at room temperature, and the solution was stirred for 24 hours. After the reaction was completed, the solvent was removed under reduced pressure to obtain a monomer crude product containing rhodamine B groups. The monomeric pure product ( ), the m...
Embodiment 2
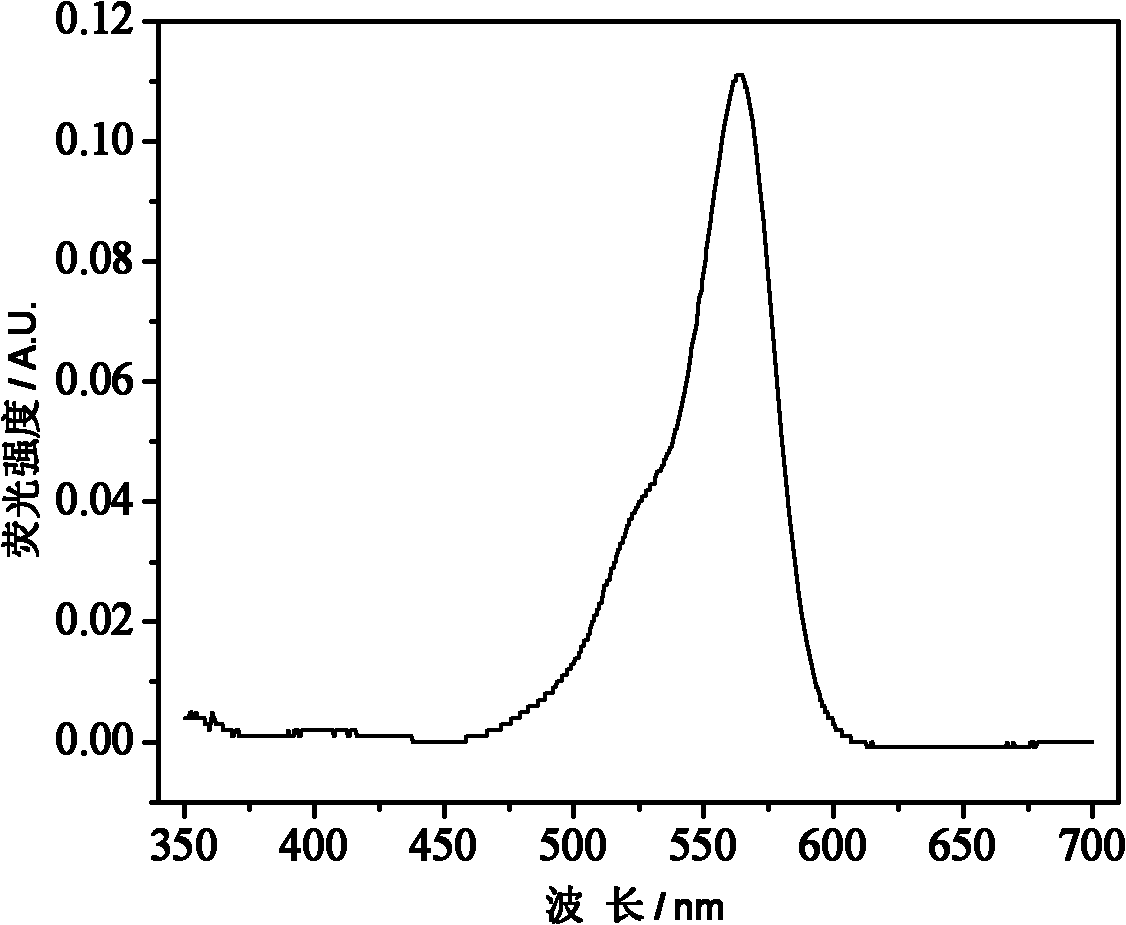
[0046] Draw the fluorescence excitation spectrum of the 0.25 mg / ml aqueous solution of the polymer PRTH1 obtained in Example 1, the abscissa is the wavelength (nm), and the ordinate is the fluorescence intensity, to obtain figure 2 ;
[0047] It can be seen from the figure that the position of the maximum excitation peak of the polymer is similar to that of Rhodamine B, which is between 550-570nm.
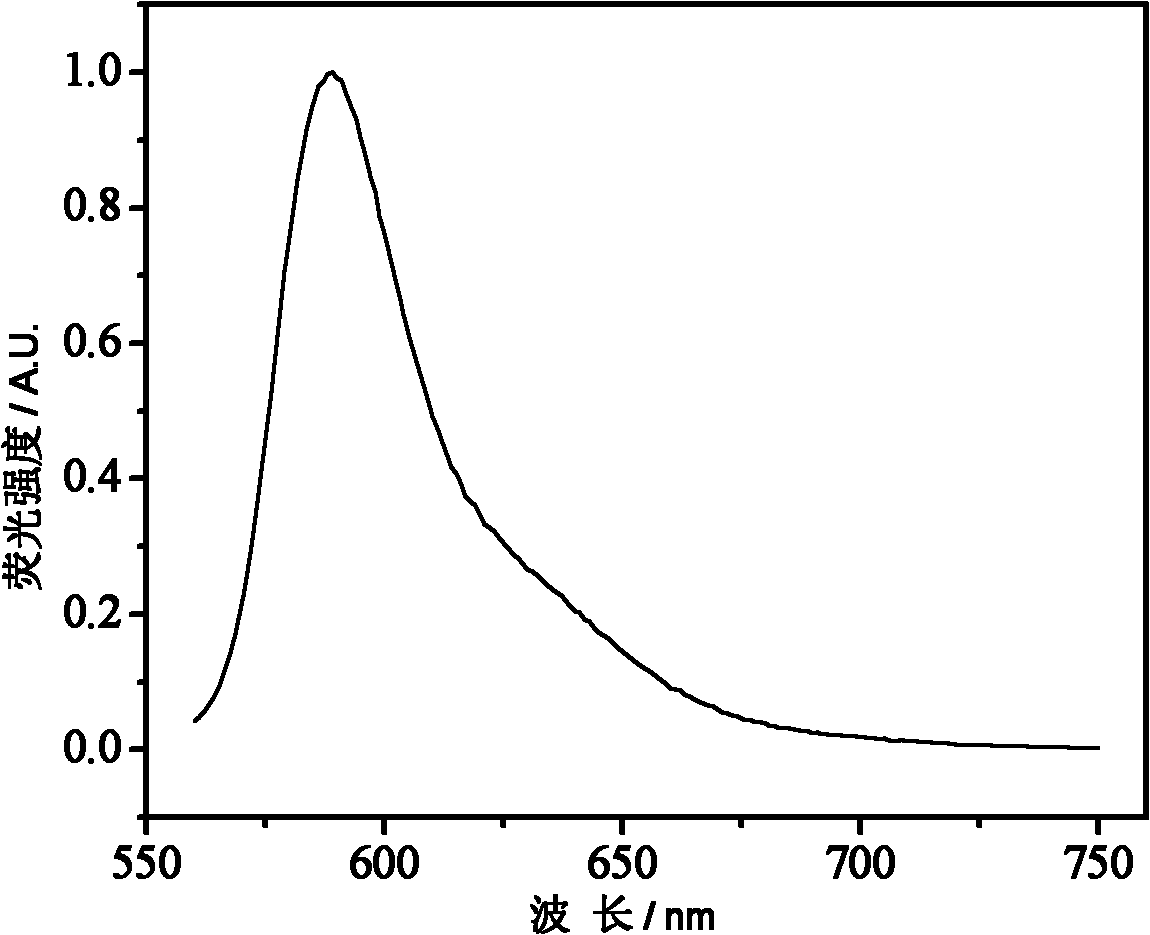
[0048] Draw the fluorescence emission spectrum of the 0.25mg / ml aqueous solution of the polymer PRTH1 gained in embodiment one, obtain image 3 , the abscissa is the wavelength (nm), and the ordinate is the fluorescence intensity.
[0049] It can be seen from the figure that the fluorescence emission peak position of this polymer is also similar to that of Rhodamine B, which is between 580-600nm.
Embodiment 3
[0051] Using the polymer PRTH1 obtained in Example 1, which can be used for fluorescence contrast imaging and isotope contrast imaging, to perform fluorescence microscopic imaging of human liver cancer cells (7402), obtain bright-field photos and fluorescent photos after polymer labeling according to the present invention ( Figure 4 ). 7402 cells were cultured in RPMI-1640 culture medium containing fluorescent polymers for 30 minutes, and then the fluorescent photos were taken, and the culture temperature was 25°C;
[0052] The results showed that red fluorescence appeared in the cells, and its fluorescence intensity was significantly higher than the background. It can be seen that polymer PRTH can enter cells more obviously and accumulate in the cytoplasm, forming a clearer fluorescence image.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com