Docetaxel liposome sterile lyophilized preparation and preparation method thereof
A freeze-dried preparation, the technology of docetaxel, which is applied to the liposome sterile freeze-dried preparation of the fat-soluble drug docetaxel and its preparation field, can solve the problem of drug leakage, inability to reconstitute liposomes, and preparation stability. investigation and other issues to achieve the effect of easy access, low price and favorable stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
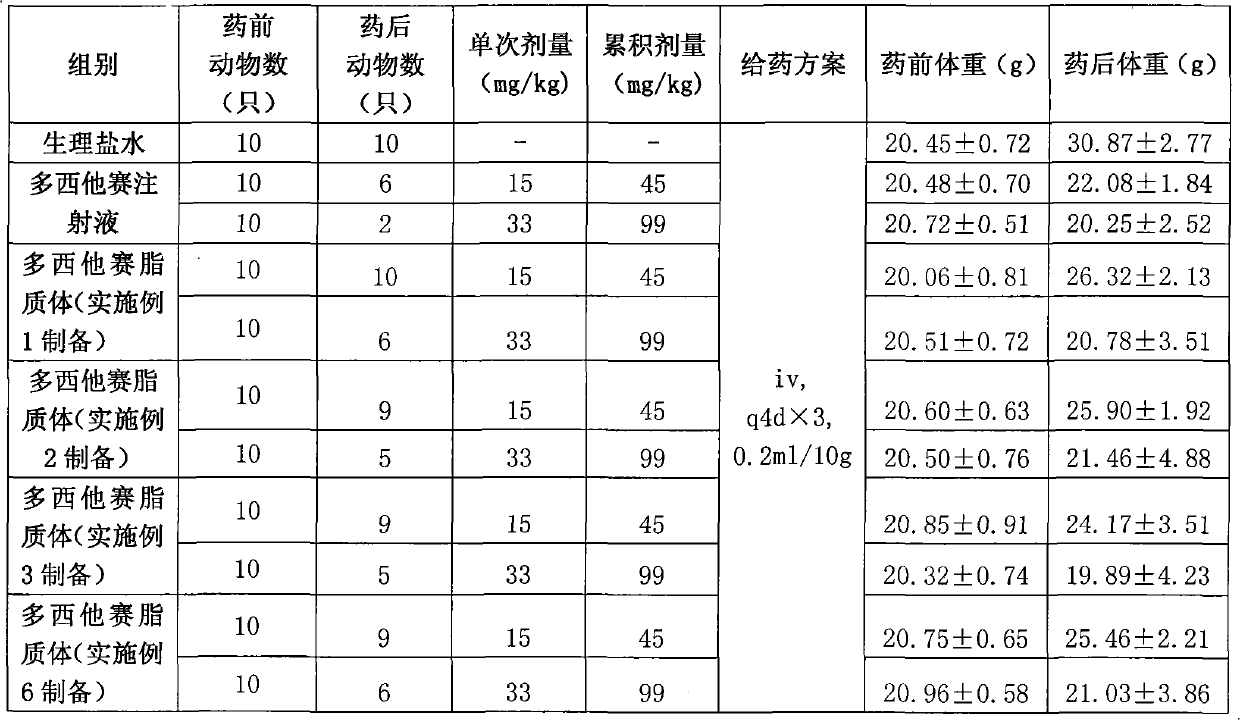
[0063] The preparation of embodiment 1 docetaxel long circulation liposome
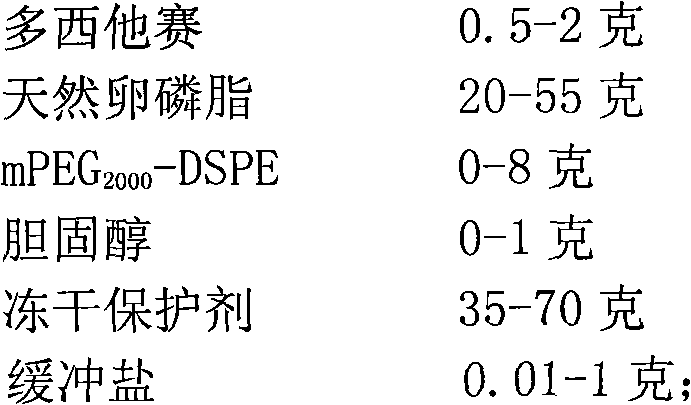
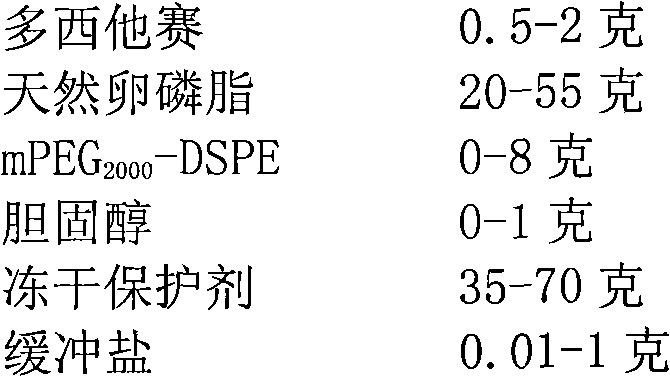
[0064] Weigh 6 grams of soybean lecithin, mPEG 2000 - Dissolve 1 g of DSPE and 0.15 g of cholesterol in 10 ml of tert-butanol, stir and dissolve at 55°C to obtain a phospholipid solution. Weigh 0.2 g of docetaxel and dissolve it in the phospholipid solution. Prepare a phosphate buffer solution with a concentration of 10 mM, adjust the pH to 4.5, add 4 grams of mannitol and 8 grams of maltose, and fully stir to dissolve. Measure 100ml of buffer solution into the phospholipid solution, and incubate with stirring at 50°C for 30 minutes to obtain a coarse suspension of liposomes. The crude suspension was added to the microfluidizer, and the 5000psi was circulated 5 times, and the 15000psi was circulated 8 times. The docetaxel long-circulating liposomes are obtained after being filtered through 0.8um, 0.45um and 0.22um filter membranes respectively.
[0065] After determination, the average particle si...
Embodiment 2
[0067] The preparation of embodiment 2 common docetaxel liposomes
[0068] Weigh 5 g of soybean lecithin and 0.1 g of cholesterol and dissolve in 10 ml of ethanol, stir and dissolve at 55° C. to obtain a phospholipid solution. Weigh 0.3 g of docetaxel and dissolve it in the phospholipid solution. Prepare a phosphate buffer solution with a concentration of 10 mM, adjust the pH to 4.5, add 3 grams of mannitol and 6 grams of maltose, and fully stir to dissolve. Measure 100ml of buffer solution into the phospholipid solution, and incubate with stirring at 50°C for 30 minutes to obtain a coarse suspension of liposomes. This crude suspension was added to the microfluidizer, 5000psi was circulated 3 times, and 15000psi was circulated 5 times. The docetaxel liposomes were obtained after being filtered through 0.8um, 0.45um and 0.22um filter membranes respectively.
[0069] After determination, the average particle size of the liposome obtained according to the above steps is 80nm, ...
Embodiment 3
[0071] The preparation of embodiment 3 docetaxel liposomes without cholesterol
[0072] Weigh 60 grams of soybean lecithin and dissolve it in 100 ml of tert-butanol, stir and dissolve at 55° C. to obtain a phospholipid solution. Weigh 1.5 g of docetaxel and dissolve it in the phospholipid solution. Prepare a phosphate buffer solution with a concentration of 10 mM, adjust the pH to 4.5, add 50 grams of mannitol and 100 grams of maltose, and fully stir to dissolve. Measure 1000ml of buffer solution into the phospholipid solution, and incubate with stirring at 50°C for 30 minutes to obtain a coarse suspension of liposomes. The crude suspension was added to the microfluidizer, and the 5000psi was circulated 5 times, and the 15000psi was circulated 8 times. The docetaxel liposomes were obtained after being filtered through 0.8um, 0.45um and 0.22um filter membranes respectively.
[0073] After determination, the average particle size of the liposome obtained according to the abov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com