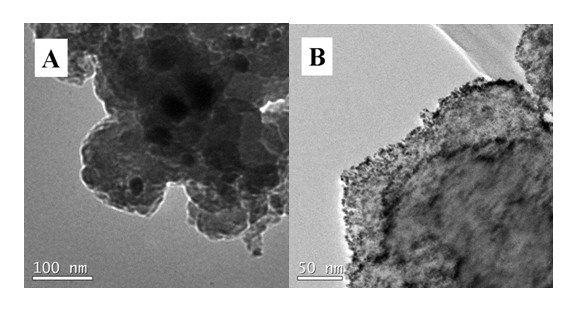
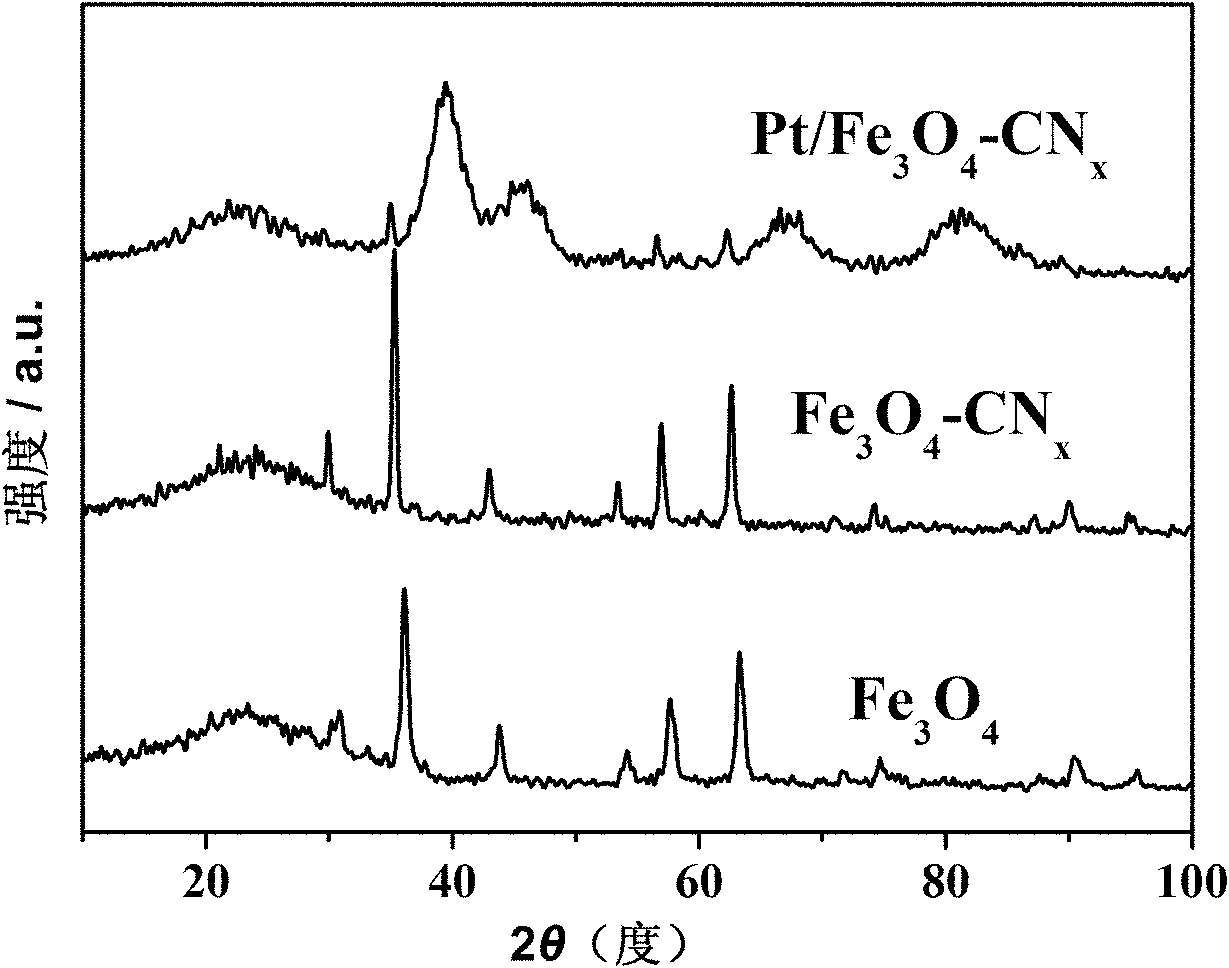
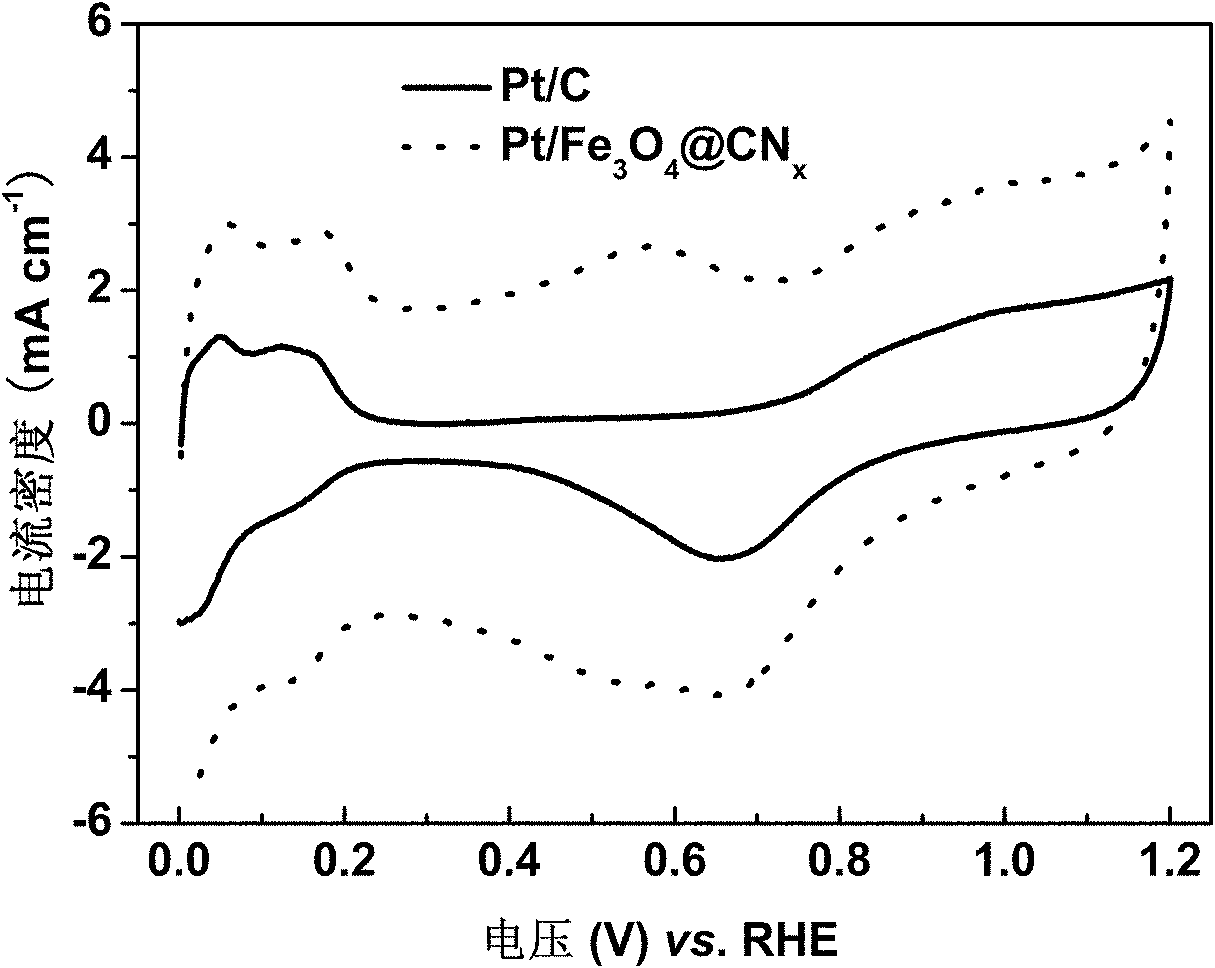
Ferroferric oxide-carbon and nitrogen composite and preparation and application thereof
A technology of ferroferric oxide and composites, applied in chemical instruments and methods, chemical/physical processes, catalyst carriers, etc., can solve the problems of easy falling off of catalyst active particles, low catalytic activity, small specific surface area, etc., and achieve good results Activity and stability, promotion of catalytic oxidation, effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a.Fe 3 o 4 -CN x Vector preparation
[0035] (1) Add 2 g of ferric chloride and 5.1 g of ferrous chloride into three times distilled water, pass through nitrogen, add ammonia water at 60 °C to make the pH of the solution = 12, and raise the temperature to 80 °C under the protection of nitrogen to react for 1 h , cooling, washing, vacuum drying for 8 h and then grinding to obtain nano-ferric oxide.
[0036] (2) Add 0.2 g of nano-iron ferric oxide into three times distilled water, ultrasonically and stir to make it fully dispersed; add 0.2 g of pyrrole monomer, 2.2 g of surfactant sodium lauryl sulfate, and then slowly drop Add the oxidant ferric chloride solution (mass concentration is 20%, containing 0.44g oxidant), and react at a temperature of 5°C for 2 hours; after the reaction is completed, suction filter, wash, and dry at 50°C to obtain ferric oxide- Precursors of carbon-nitrogen complexes.
[0037] (3) Heat treatment of ferric oxide-carbon nitrogen composite...
Embodiment 2
[0042] a.Fe 3 o 4 -CN x Vector preparation
[0043] (1) Add 2.3 g of ferric chloride and 5.8 g of ferrous chloride into three times distilled water, blow in nitrogen, add ammonia water at 60 °C to make the pH of the solution = 11, raise the temperature to 60 °C under nitrogen protection and keep for 3 h , cooling, washing, vacuum drying for 10 h and then grinding to obtain nanometer ferric oxide.
[0044] (2) Add 0.23 g of nanometer iron ferric oxide into triple distilled water, ultrasonically stir to make it fully dispersed, add 0.26 g of pyrrole monomer, and 2.4 g of surfactant octadecyltrimethylammonium bromide in sequence , and then slowly dropwise added the oxidizing agent ferric chloride solution (mass concentration: 25%, containing 0.55 g of oxidizing agent), and reacted at a temperature of 3 °C for 2 hours. After the reaction was completed, suction filtered, washed, and dried at 60 °C to obtain four Ferric oxide-carbon nitrogen complex precursor.
[0045] (3) The...
Embodiment 3
[0050] a.Fe 3 o 4 -CN x Vector preparation
[0051] (1) Add 3 g of ferric chloride and 6.8 g of ferrous chloride into three times distilled water, pass through nitrogen, add ammonia water at 85 °C to make the pH value of the solution = 9, raise the temperature to 75 °C under nitrogen protection and keep for 3 h, cooling, washing, vacuum drying for 8 h, and grinding to obtain nano-ferric oxide.
[0052] (2) Add 0.26 g of nanometer iron ferric oxide into triple distilled water, ultrasonically and stir to make it fully dispersed; add 0.3 g of pyrrole monomer, 2.7 g of surfactant ammonium oleate in sequence, and then slowly add the oxidant dropwise over Ammonium sulfate (mass concentration: 25%, containing 0.72 g oxidant), reacted at 0 °C for 5 hours; after the reaction was completed, suction filtered, washed, dried at 50 °C, ferric oxide-carbon nitrogen composite precursor.
[0053] (3) The ferroferric oxide-carbon-nitrogen composite precursor was heat-treated at 650 °C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com