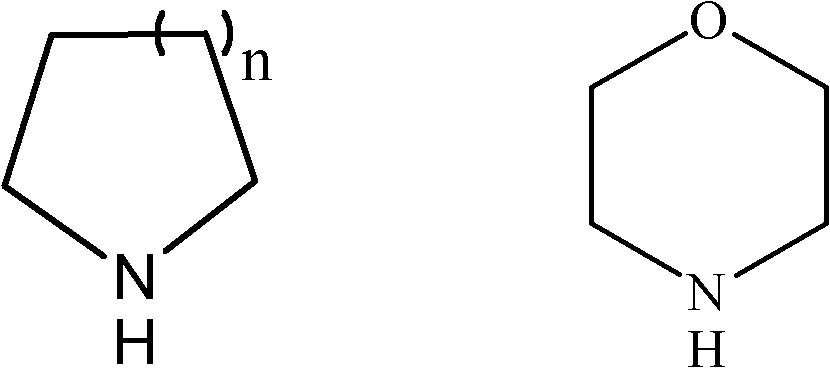
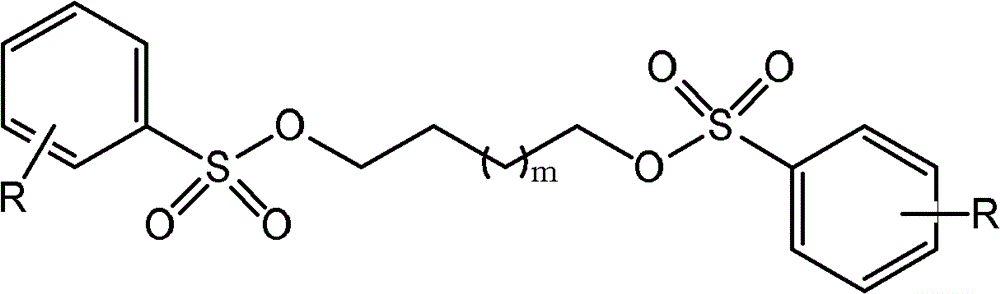
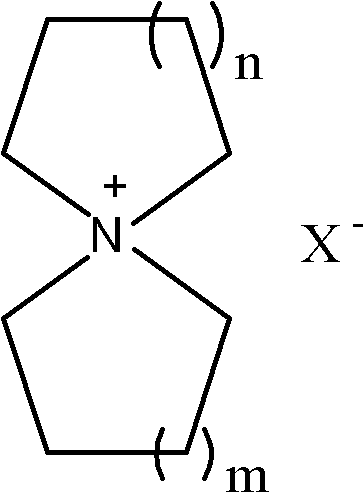Preparation method of spiro quaternary ammonium salt for organic electrolyte of super capacitor
A technology of spirocyclic quaternary ammonium salt and organic electrolyte, which is applied in the direction of organic chemistry, can solve the problems of low yield and difficult to meet the requirements of electrochemical products, and achieve the effect of high yield, good quality and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of spiro-1,1'-pyrrole quaternary ammonium salt
[0039] 1.1 Preparation of p-toluenesulfonic acid spiro-1,1'-pyrrole quaternary ammonium salt
[0040] In a 2-liter three-necked reaction flask, tetrahydropyrrole (35.5 grams, 0.5 moles) was added dropwise to 1,4-butanediol di-p-toluenesulfonate (238.8 grams, 0.6 moles) and potassium carbonate (103.5 g, 0.75 mol) in deionized water (750 ml), reacted at 25°C for 5 hours, and then heated to reflux for 8 hours. Concentrate under reduced pressure, add ethanol to the obtained residue, and filter. The filtrate was concentrated and recrystallized from ethanol to obtain 130.7 g of spiro-1,1'-pyrrole quaternary ammonium p-toluenesulfonate with a yield of 88%.
[0041] The structural analysis is as follows:
[0042] The NMR analysis results are: 1 H NMR (400MHz, D 2 O): δ=2.30-2.35(m, 8H, 4xCH 2 ), 2.44 (s, 3H, CH 3 ), 3.61-3.66 (m, 8H, 4xCH 2 ), 7.51(d, 2H), 7.87(d, 2H).
[0043] 1.2 Preparation of spiro-1,1’-p...
Embodiment 2
[0061] Preparation of spiro-1,1'-pyrrole quaternary ammonium salt
[0062] 2.1 Preparation of spiro-1,1'-pyrrole quaternary ammonium salt of methanesulfonate
[0063] In a 2-liter three-necked reaction flask, tetrahydropyrrole (35.5 grams, 0.5 moles) was added dropwise to 1,4-butanediol dimethylsulfonate (128.4 grams, 0.6 moles) and sodium carbonate ( 91.5 g, 0.75 mol) of deionized water (800 ml), reacted at 25° C. for 6 hours, and then heated to reflux for 10 hours. Concentrate under reduced pressure, add ethanol to the obtained residue, and filter. The filtrate was concentrated and recrystallized from ethanol to obtain 93.9 g of spiro-1,1'-pyrrole quaternary ammonium methanesulfonate with a yield of 85%.
[0064] The structural analysis is as follows:
[0065] The NMR analysis results are: 1 H NMR (400MHz, D 2 O): δ=2.33-2.37(m, 8H, 4xCH 2 ), 2.96 (s, 3H, CH 3 ), 3.62-3.67(m, 8H, 4xCH 2 ).
[0066] 2.2 Preparation of spiro-1,1’-pyrrole quaternary ammonium tetrafluor...
Embodiment 3
[0077] Preparation of spiro(piperidine-1,1'-pyrrole) quaternary ammonium salt
[0078] 3.1 Preparation of spiro(piperidine-1,1'-pyrrole) quaternary ammonium salt of p-toluenesulfonate
[0079] In a 2-liter three-necked reaction flask, under stirring, hexahydropiperidine (51.0 grams, 0.6 moles) was added dropwise to 1,4-butanediol di-p-toluenesulfonate (238.8 grams, 0.6 moles) and sodium carbonate (91.5 g, 0.75 mol) in deionized water (800 ml), reacted at 25° C. for 5 hours, and then heated to reflux for 10 hours. Concentrate under reduced pressure, add ethanol to the obtained residue, and filter. The filtrate was concentrated and recrystallized from ethanol to obtain 149.3 g of p-toluenesulfonate spiro(piperidine-1,1'-pyrrole) quaternary ammonium salt with a yield of 80%.
[0080] The structural analysis is as follows:
[0081] The NMR analysis results are: 1 H NMR (400MHz, D 2 O): δ=1.71-1.76(m, 2H), 1.91-1.96(m, 4H, 2xCH 2 ), 2.30-2.35(m, 4H, 2xCH 2 ), 2.46(s, 3H, CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com