Porous polymer material and preparation method thereof
A porous polymer, aromatic compound technology, applied in the directions of alkali metal compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of limited styrene monomers, increase production costs, The problems of high energy consumption, etc., achieve the effects of wide applicability, mild reaction conditions, and simple synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
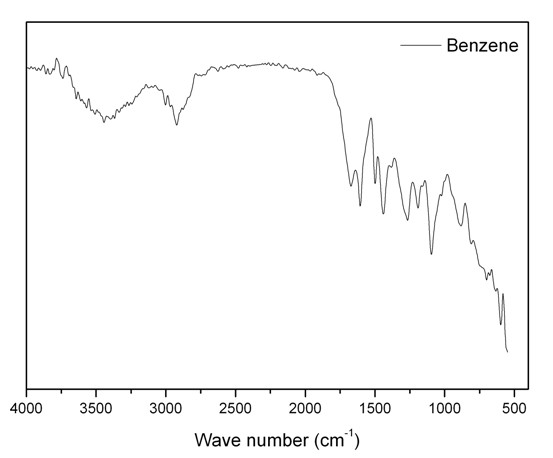
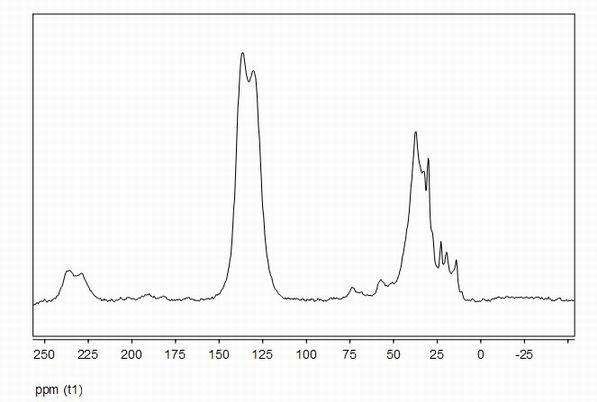
[0024] At room temperature, add 20ml of 1,2-dichloroethane, 1.56g of benzene and 4.56g of dimethoxymethane into a three-necked flask equipped with a reflux condenser and a thermometer, and stir magnetically for 0.2 hours. Then add 6.50 g of anhydrous ferric chloride, raise the temperature to 45° C., stir magnetically at this temperature for 5 hours, then raise the temperature to 80° C., and stir magnetically at this temperature for 18 hours. During the above process, magnetic stirring was maintained. The reaction product was filtered to obtain a brown solid crude product, which was washed three times with ether and methanol, and then extracted with methanol in a Soxhlet extractor for 24 hours. Finally dry under reduced pressure to obtain the porous polymer material, its infrared spectrum is shown in the attached figure 1 , at 1600cm -1 , 1500cm -1 and 1450cm -1 The infrared peaks prove that the porous polymer has an aromatic ring structure; 13 C solid NMR spectrum see att...
Embodiment 2
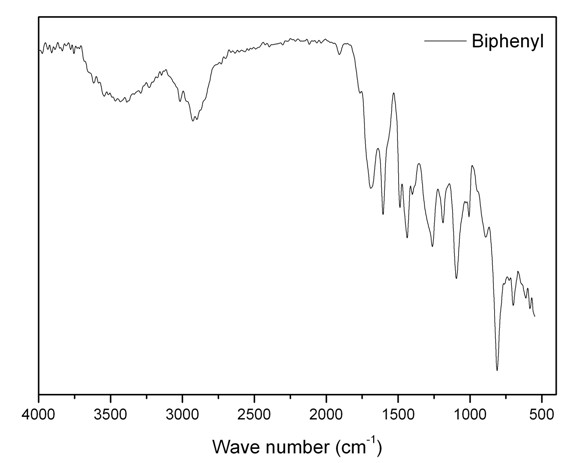
[0026] At room temperature, add 20ml of 1,2-dichloroethane, 3.08g of biphenyl and 3.04g of dimethoxymethane into a three-necked flask equipped with a reflux condenser and a thermometer, and stir magnetically for 0.5 hours. Then add 6.50 g of anhydrous ferric chloride, raise the temperature to 45° C., stir magnetically at this temperature for 5 hours, then raise the temperature to 80° C., and stir magnetically at this temperature for 18 hours. During the above process, magnetic stirring was maintained. The reaction product was filtered to obtain a brown solid crude product, which was washed three times with ether and methanol, and then extracted with methanol in a Soxhlet extractor for 24 hours. Finally dry under reduced pressure to obtain the porous polymer material, its infrared spectrum is shown in the attached image 3 , at 1600cm -1 , 1500cm -1 and 1450cm -1 The infrared peaks prove that the porous polymer has an aromatic ring structure;13 C solid NMR spectrum see atta...
Embodiment 3
[0028] At room temperature, add 20ml of 1,2-dichloroethane, 1.86g of toluene and 3.04g of chloromethyl methyl ether into a three-neck flask equipped with a reflux condenser and a thermometer, and stir magnetically for 0.5 hours. Then add 6.50 g of anhydrous ferric chloride, raise the temperature to 45° C., stir magnetically at this temperature for 5 hours, then raise the temperature to 80° C., and stir magnetically at this temperature for 18 hours. During the above process, magnetic stirring was maintained. The reaction product was filtered to obtain a brown solid crude product, which was washed three times with ether and methanol, and then extracted with methanol in a Soxhlet extractor for 24 hours. Finally dry under reduced pressure to obtain the porous polymer material, its infrared spectrum is shown in the attached Figure 5 , at 1600cm -1 , 1500cm -1 and 1450cm -1 The infrared peaks prove that the porous polymer has an aromatic ring structure; 13 C solid NMR spectrum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com