Preparation method of environmentally-friendly aqueous polyurethane adhesive
A water-based polyurethane and adhesive technology, applied in the direction of polyurea/polyurethane adhesives, adhesives, adhesive types, etc., can solve the problems of inconvenience for large-scale production, increase production costs, and difficulty in operation, and achieve energy saving, The initial viscosity is strong, and the effect of not easy to agglomerate and precipitate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
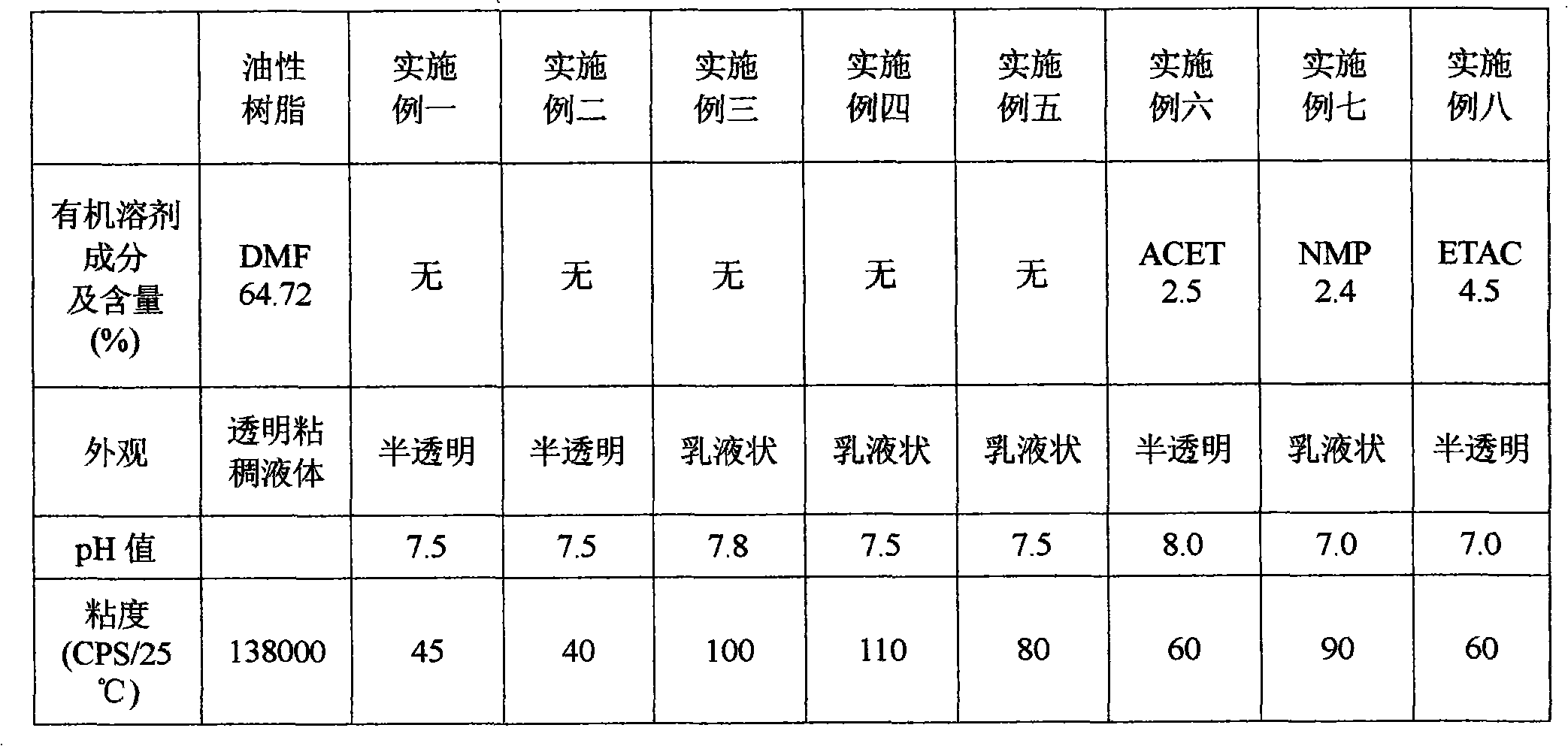
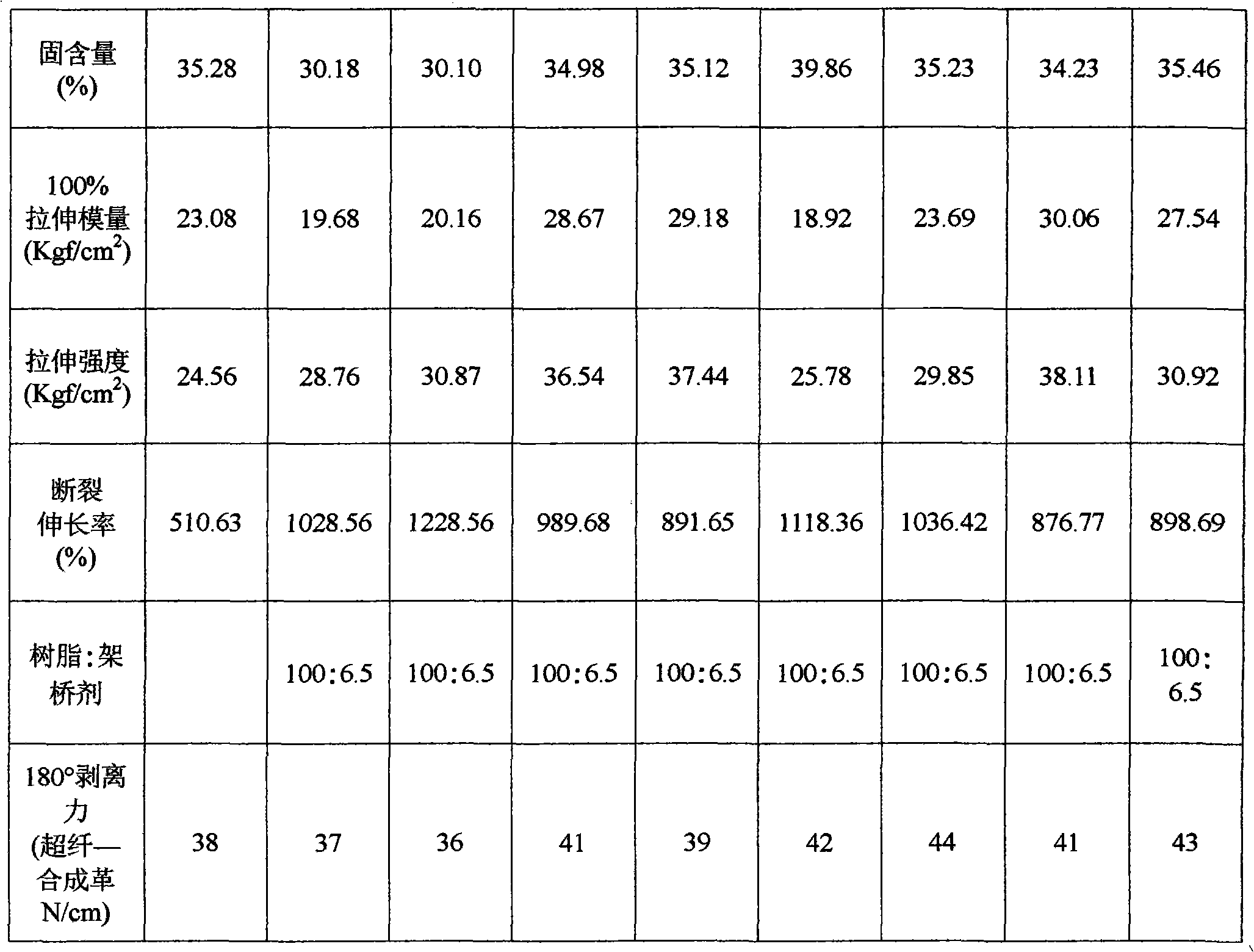
[0022] The raw material composition of the preparation method of this environment-friendly water-based polyurethane adhesive is calculated in mass percentage as:
[0023] Number-average molecular weight is 10-50% of polymerized diols with a molecular weight of 500-5000,
[0024] The number average molecular weight is 0.1~10% of the hydrophobic chain extender of 61~400,
[0025] Internal crosslinking agent 0~5%,
[0026] Hydrophilic chain extender 0.1~10%,
[0027] Diisocyanate 2~30%,
[0028] Catalyst 0.001~0.1%,
[0029] Organic solvent 0~10%,
[0030] Neutralizer 0.2~5%;
[0031] Deionized water 40-80%;
[0032] Its preparation process is as follows:
[0033] (a) Add polymeric diol, hydrophobic chain extender, internal crosslinking agent, and hydrophilic chain extender into the reaction kettle, raise the temperature to 110-120°C under the protection of nitrogen, and vacuum dehydrate for 15-30 minutes ;
[0034] (b) Cool to 50-60°C, add diisocyanate, the molar ratio ...
Embodiment 1
[0049] 1. Polyoxypropylene glycol 160g (number average molecular weight is 2000), hydrophobic small molecule chain extender neopentyl glycol 4.16g, internal crosslinking agent trimethylolpropane 0.54g, hydrophilic chain extender II Add 8.58 g of methylolpropionic acid (DMPA) into a four-port reaction kettle equipped with a thermometer, a condenser, and a stirring device. Under the protection of nitrogen, heat up to 120°C, vacuum dehydrate for 30 minutes, cool to 60°C, and add 45.91g isophorone diisocyanate (IPDI) (the molar ratio of diisocyanate to active hydrogen compound is 1.1:1), react at 90°C for 2 hours, then lower the temperature to 80°C, add 0.05g tetraisopropyl titanate Alcohol ester, reacted for 3 hours.
[0050] 2. Under the action of high-speed shear force (stirring speed at 2000r / min), the aqueous solution of neutralizing agent triethylamine (triethylamine 3.88g, deionized water 520g) is added in the reaction kettle, neutralizing agent three The molar ratio of et...
Embodiment 2
[0052] 1. Add 160g of polyoxyethylene diol (the number average molecular weight is 2000), 4.16g of neopentyl glycol, 0.54g of trimethylolpropane, and 8.58g of dimethylol propionic acid into the reactor, under the protection of nitrogen , heat up to 120°C, vacuum dehydrate for 30 minutes, cool to 60°C, add 45.91g of isophorone diisocyanate (the molar ratio of diisocyanate to active hydrogen compound is 1.1:1), react at 90°C for 2 hours, and then Lower the temperature to 80° C., add 0.05 g of tetraisopropanol titanate, and react for 3 hours.
[0053] 2. Under the effect of high-speed shear force (stirring speed at 2000r / min), the aqueous solution of triethylamine (3.88g of triethylamine, 520g of deionized water) is added in the reaction kettle, stirred for 15 minutes, and the obtained viscosity is 40CPS / 25℃, solid content 30.10%, pH value 7.5, translucent water-based polyurethane adhesive without organic solvents.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com