Lentinan granule as well as preparation method and use of lentinan granule
A technology of lentinan and granules, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of affecting growth and quality, inconvenient operation, and large irritation of animal organisms, etc. Stable content, reduced stress response, less hygroscopic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
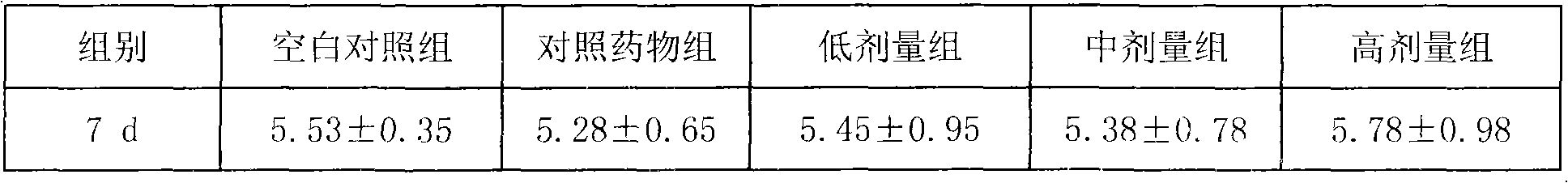
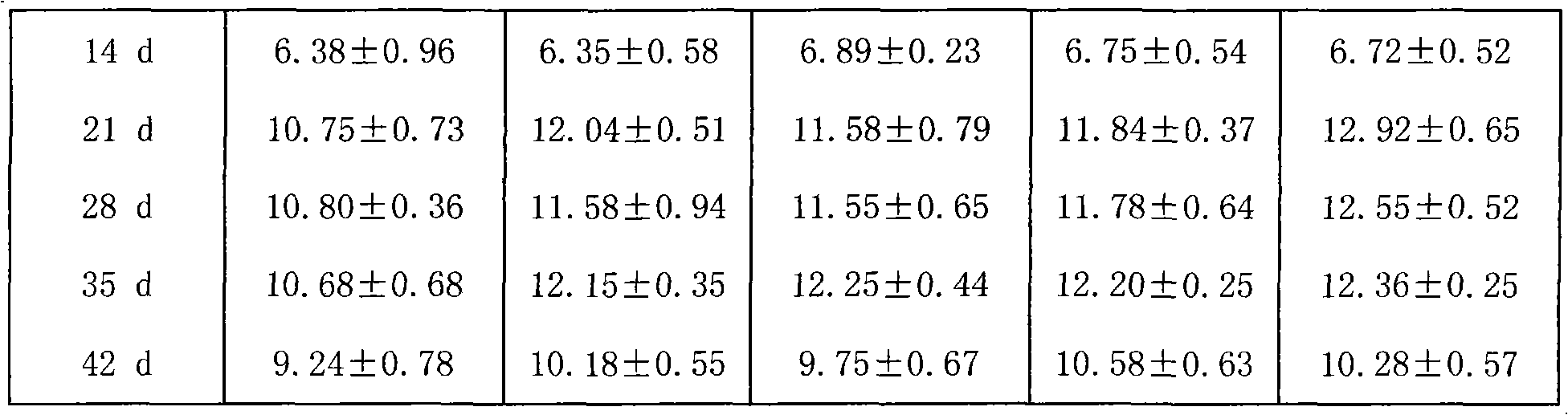
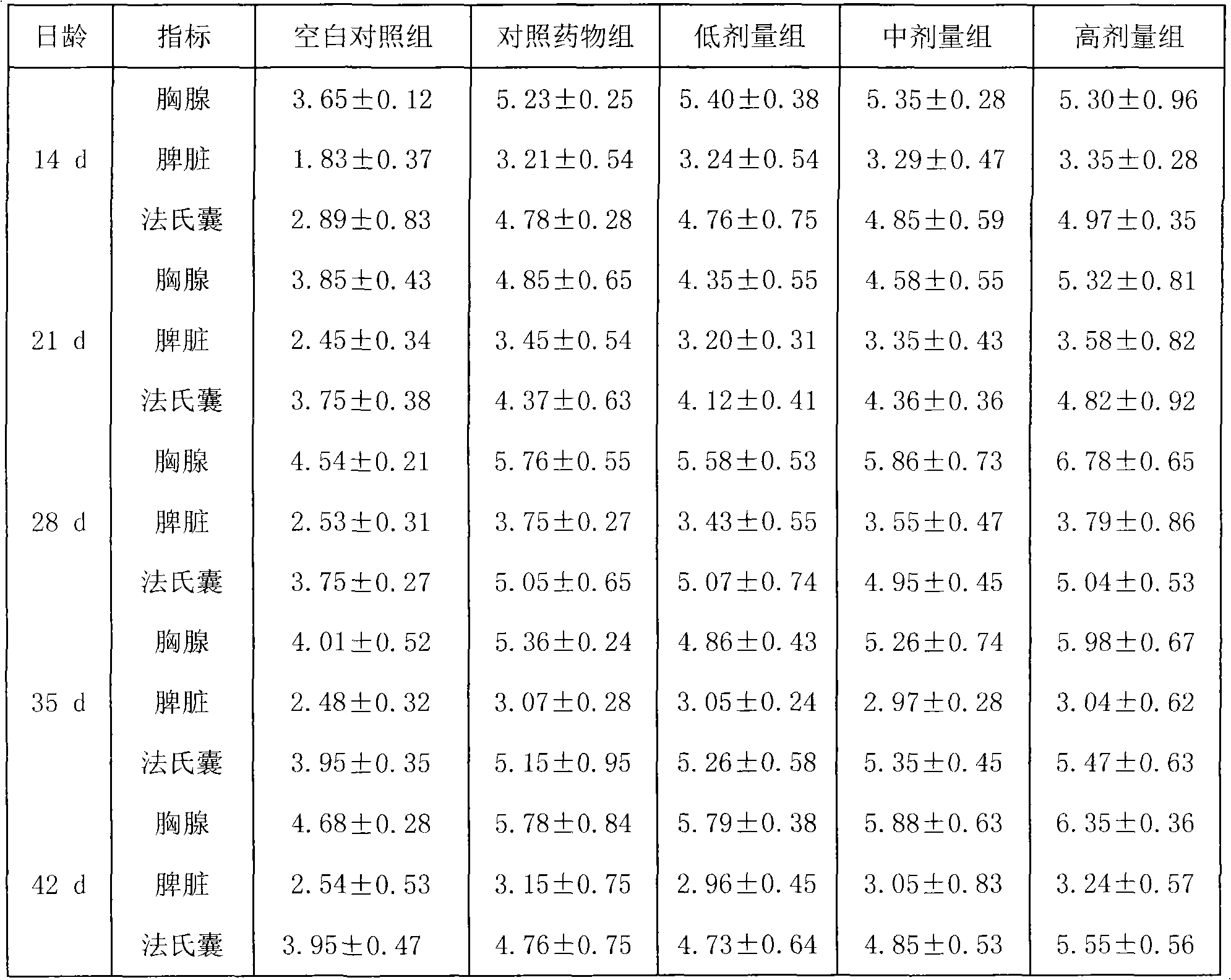
Examples
Embodiment 1
[0023] The preparation of embodiment 1 lentinan granules
[0024] Prepare as follows:
[0025] (1), Lentinan (purchased from Fuzhou Meifeng Pharmaceutical Factory of Jinling Pharmaceutical Co., Ltd., the specification is 10g / bottle (plastic bottle), and the approval number is Z1092011, the same below), powdered sugar (purchased from Shandong Xiangrui Pharmaceutical Co., Ltd., the specification is 25kg / bag, and the approval number is Guoyao Zhunzi H20013420, the same below), hypromellose (purchased from Anhui Shanhe Pharmaceutical Excipients Co., Ltd., the specification is 25kg / bag, approved Document number is Wanyao Zhunzi (2002) No. F0003), etc., and then dried and pulverized; lentinan was passed through a 100-mesh sieve, and sugar powder and hypromellose were passed through a 80-mesh sieve for later use.
[0026] (2) Put 50g of lentinan and 600g of powdered sugar in a mixer, then gradually add 300g of hydroxypropyl methylcellulose and 50g of water, and mix well to make a so...
Embodiment 2
[0027] The preparation of embodiment 2 lentinan granules
[0028] Prepare as follows:
[0029] (1), Lentinan, mannitol (purchased from Jiangsu Huanghai Pharmaceutical Co., Ltd., the specification is 25kg / bag, and the approval number is Guoyao Zhunzi H32022586), dextrin (purchased from Anhui Shanhe Pharmaceutical Excipients Co., Ltd., specification It is 25kg / bag, and the approval number is Wanyao Zhunzi (2002) No. F0001), etc., are dried separately, and pulverized; Lentinan is passed through a 100-mesh sieve, and mannitol and dextrin are passed through a 80-mesh sieve for later use;
[0030] (2) Put 30g of lentinan and 750g of mannitol in a mixer, gradually add 200g of dextrin and 20g of water, mix well to make a soft material; pass the soft material through a 16-mesh sieve to make wet granules, and then drum at 40°C After air-drying, use 20-mesh whole grains to obtain lentinan granules.
Embodiment 3
[0031] The preparation of embodiment 3 lentinan granules
[0032] Prepare as follows:
[0033] (1), Lentinan, powdered sugar, povidone K30 (purchased from Zhejiang Maoyuan Pharmaceutical Co., Ltd., the specification is 25kg / barrel, approval number Wenzhe Yao Zhun Zi (2004) No. 224602, the same below) Dried and crushed separately; lentinan was passed through a 100-mesh sieve, sugar powder and povidone K30 were passed through a 80-mesh sieve for later use;
[0034] (2) Put 50g of lentinan and 700g of powdered sugar in a mixer, gradually add 200g of povidone K30 and 50g of water, and mix well to make a soft material; pass the soft material through a 16-mesh sieve to make wet granules, and then 40°C After blast drying, use 20-mesh whole grains to obtain lentinan granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com