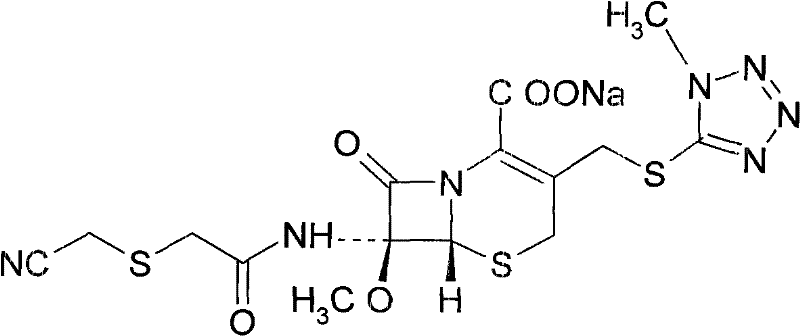
Cefmetazole sodium liposome freeze-dried preparation and preparation method
A technology of cefmetazole sodium lipid and freeze-dried preparations, which is applied in the field of cefmetazole sodium liposome freeze-dried preparations and preparations, can solve the problem of temperature, light, and humidity instability, which can easily cause quality changes and increase the safety of clinical medication Sex and other issues, to achieve a good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Cefmetazole Sodium 1.0 weight unit
[0026] Sodium chloride 1.5 weight units
[0027] Egg yolk lecithin, distearic acid lecithin, soybean lecithin 1:1 mix 2.5 weight units
[0028] Mannitol 1.0 weight unit
[0029] Sodium hydrogen phosphate 0.5~3.0 weight units
[0030] Weigh the prescription amount of cefmetazole sodium, sodium chloride, and mannitol, mix and dissolve in water, stir evenly, and set aside; weigh an appropriate amount of sodium hydrogen phosphate to prepare a 10% buffer salt solution, and mix the prescription amount of egg yolk lecithin and double hard Fatty acid lecithin and soybean lecithin are dispersed and dissolved, dried under reduced pressure to form a blank film material, added to the above aqueous solution, and ultrasonically processed to a cefmetazole sodium liposome suspension with the desired particle size and uniformity; Containing, filling, and vacuum freeze-drying to obtain cefmetazole sodium liposome freeze-dried preparation.
Embodiment 2
[0032] Cefmetazole Sodium 1.0 weight unit
[0033] Sodium chloride 1.5 weight units
[0034] Lecithin Dipalmitate 5.0 weight units
[0035] Mannitol 1.0 weight unit
[0036] Calcium hydrogen phosphate 0.5~3.0 weight units
[0037] Weigh the prescription amount of Cefmetazole Sodium, Sodium Chloride, and Mannitol, mix them and dissolve in water, stir evenly, and set aside; Weigh an appropriate amount of calcium hydrogen phosphate to prepare a 10% buffer salt solution, and mix the prescription amount of Lecithin with double palmitate Disperse and dissolve, dry under reduced pressure to form a blank film material, add it to the above aqueous solution, and ultrasonically treat it to the required particle size and uniformity of the cefmetazole sodium liposome suspension; constant volume, filling, and vacuum freezing After drying, the lyophilized preparation of cefmetazole sodium liposome is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com